Rolf Knippers protocols J
advertisement

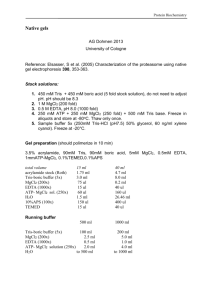
Isolation of Chromatin Fractions Rolf Knippers protocols in J. Biol Chem. 2001 276, 6337-6342 J. Biol. Chem. 1998 273, 24543-24549. first described by R.Hancock J. Mol. Biol. 1974 86, 649-663. modified by E. Benevolenskaya 7/22/2003 From a subconfluent p100 plate: 1) Isolation with Micrococcal nuclease treatment. Cells are washed on plates with 5 ml of ice-cold Buffer A (10mM Hepes/K+, pH 7.5, 20 mM KCl, 0.25 mM EDTA). The cells were incubated for 10 min at 40C with 1 ml of Buffer A (5 mM MgCl2 was added in some applications plus proteinase inhibitors), inducing swelling and disruption of the cytoplasmic membrane. The released nuclei were concentrated at 700 g 4 min and resuspended in 50-100 mkl of 10mM Hepes/K+, pH 7.5, 70 mM NaCl, 20 mM KCl, 5 mM MgCl2, 2 mM CaCl2. Micrococcal nuclease (Boehringer Mannheim) was added in amount of 0.03 unit per p100 plate of Saos-2 cells and nuclei were digested for 5 min at 16C (this degree of underdigestion is enough for release of pRB and RBP2 proteins). After the digestion, suspension was immediately cooled on ice and centrifuged at 1000 rpm for 4 min. The supernatant is S1 fraction. Reaction was stopped and nuclei lysed by incubating in 50-100 mkl of 3 mM EDTA, 0.25mM EGTA, 1 mM DTT, proteinase inhibitors, for 15 min on ice. Suspension was centrifuged at 1500 rpm for 4 min. The supernatant is fraction S2. Pellet (P) was solubilized in 100 mkl of 50 mM Tris-HCl pH 8, 10mM EDTA, 1% SDS, and diluted 1:10 for IP in PBS LSB (PBS, 25 mM MgCl2, 10 mM EDTA, 10% glycerol, 1 mM DTT, that has been used for detection of pRB-Brm interactions). Insoluble material was removed by centrifugation. If not for IP, P was solubilized by boiling in 50 mM Tris-HCl pH 6.8, 2% SDS. Digested chromatin of S1 and S2 was used for IP in page 1 Draft 8/13/01 PG and PB PBS LSB or deproteinized in 0.5% SDS, 2000 mkg/ml proteinase K for 30 min at 37C and investigated by agarose gel electrophoreses and ethidium bromide staining. IPs were washed in PBS LSB containing 3% glycerol. 2) Isolation by NaCl extraction. After collecting Buffer A- released nuclei, nuclei were lysed in 500 mkl of 10mM Hepes/K+, pH 7.5, 70 mM NaCl, 20 mM KCl, 5 mM MgCl2, 2 mM CaCl2, 0.3%-0.5% NP-40, proteinase inhibitors, for several minutes and loaded on a 4.5 ml sucrose cushion (0.5 mM Tris-HCl, pH 8.5, 0.1M sucrose). Chromatin bodies were centrifuged through the sucrose cushion for 10 min at 3 000 g. Chromatin bodies were resuspended in 100 mkl of 20 mM Hepes/K+, pH 7.5, 0.5 mM MgCl2, 0.3 M sucrose and NaCl of respective concentration. I used 0.15M, 0.25 M, 0.45 M NaCl sequential extraction. Chromatin bodies were incubated in each solution 10 min and centrifuged at 3 000 g for 3 min. Load total nuclear fraction (before the sucrose cushion), total chromatin (after the sucrose cushion), and sequential NaCl fractions on the gel to examine by Western blotting the extraction conditions. page 2 Draft 8/13/01 PG and PB