Acquisition of Samples from Healthy Human Donors
advertisement

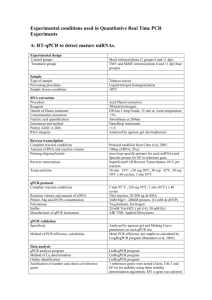
Supplemental Methods. Acquisition of samples from normal human donors The CD34+ cells from bone marrows (BMCD34+, N = 10), CD34+ cells from peripheral blood stem cell products (PBCD34+, N = 11), and naïve T-cells form peripheral blood (T-cells, N = 44) were either obtained from volunteer donors at the Fred Hutchinson Cancer Research Center (FHCRC), Adult Changes In Thought Cohort from Group Health Cooperative (Seattle, WA),1 or purchased from commercially available vendors (Cambrex, Rutherford, NJ or AllCells, Emeryville, CA). Donors with a history of previous radiation, chemotherapy, or hematologic malignancies were excluded. In the case of the BMCD34+ and PBCD34+ samples, donors of all ages were included in order to obtain adequate sample numbers for analyses. For the T-cells samples, there was a larger pool of potential donors, and thus, two populations were selected: younger (age ≤ 30) and older (age 70) adults. All samples were obtained under Institutional Review Board (IRB) approved protocols according to the Declaration of Helsinki. The demographics and age of each donor is provided in Supplement 2. Anti-CD34 immunomagnetic beads CD34+ cells (Miltenyi Biotec, Auburn, CA) were used to select highly purified populations of CD34+ cells as previously described.2,3 Percentage of viable CD34+ cells was determined to be 88% (range 88% – 98%). The percent of viable CD34 cells for the BMCD34+ and PBCD34+ did not significantly correlate with age (R = 0.35, p = 0.35 and R = 0.22, p = 0.55, respectively). Some of PBCD34+ samples with adequate material were further purified into a highly enriched population of PBCD34+/38- cells by flow-cytometric sorting.4 Naïve T-cells were selected from fresh peripheral blood samples following mononuclear cell separation with Hypague-Ficoll via dual negative selection with a CD4+ T-cell Isolation Kit II (No. 130-091-155, Miltenyi Biotec Inc., Auburn CA) and CD45 RO+ Microbeads (No. 130-046-001, Miltenyi Biotec Inc.) as per manufacturer’s recommendations (www.miltenyibiotec.com). The naïve T-cells underwent expansion using Dynabeads CD3/CD28 T-cell expander beads (No. 111.31, Invitrogen, Carlsbad, CA) as per manufacturer’s recommendations (www.invitrogen.com) and as previously described.5 The purity of naïve T-cells prior to expansion was 90%. The mean number of days of T-cell expansion (MDE) prior to harvest and RNA extraction was 8.9 days. There were no significant difference in the MDE between the samples from the younger and older subjects (T-cells in microarray studies, young 9.3 MDE older 10.00 MDE, p = 0.30 and T-cells in Q-RT/PCR studies, young 8.0 MDEs. older 8.4 MDE, p = 1.00). RNA extraction and hybridization to microarrays RNA was extracted from CD34+ cells and T-cells using TRIzol reagent (Invitrogen), and RNA quality was analyzed on a HP 2100 Bioanalyzer (Agilent Technologies, Palo Alto, CA).6,7 RNA was not pooled, treating each sample as an independent variable. Five micrograms of total RNA from BMCD34+ and Tcells was prepared for microarrays using a Enzo BioArrayTM High YieldTM RNA Transcript Labeling Kit (Enzo Life Technologies, Farmingdale, NY) according to the Eukaryotic Target Labeling protocol as described in GeneChipTM Expression Analyses Technical Manual (Affymetrix, Santa Clara, CA). 7,8 For the PBCD34+/38- arrays, the single-stranded linear amplification protocol was used to amplify 100 nanograms of total RNA, which then biotin-labeled using Enzo BioArrayTM High YieldTM RNA Transcript Labeling Kit (Enzo Life Technologies).7 A total of 15 µg of biotin-labeled, fragmented target was hybridized to HG-U133A arrays (Affymetrix).7,8 Microarrays and analyses of human hematopoietic blood cells DAT files were generated with GCOS 1.2.1 software (Affymetrix). Target signals were scaled to 500, and CEL files were generated using MAS 5.0 software (Affymetrix).6 All arrays met previously published quality control standards.6,8-10 Robust multi-array average (gcRMA) was used to normalize data and to generate log2 expression values from CEL files.6,11,12 Normalization was performed separately for each data set (e.g. BMCD34+, PBCD34+/38-, and Tcells). Log2 expression values were analyzed in GenePlus software (Enodar Biologic, Seattle, WA).6 Demographic data and CEL files are available at the Gene Expression Omnibus (www.ncbi.nlm.nih.gov/geo). Analyses for age-associated expression changes were performed independently in BMCD34+ (N = 8 donors), PBCD34+38- (N = 4) and expanded T-cell (N = 18 donors, 8 younger and 10 older).13 In the case of the BMCD34+ and PBCD34+38- analyses, age was treated as a continuous variable. In the case of the T-cell analyses, a two group comparison analysis (young, age ≤ 30 vs. older, age > 70) was performed. For the BMCD34+ and T-cell studies, analyses controlled for gender. In the case of the PBCD34+38-, there were no females in the data set. Age-associated expression changes with Z-values 4.75 or ≤ -4.75 were deemed to be statistically significant, which has been established as statistical cut-off for similar array studies.14,15 Microarray analyses of murine hematopoietic stem cells Studies of age-associated expression changes were also performed using microarray data of murine hematopoietic stem cells (HSCs). The murine Rossi et al. data set (GEO, GEO accession: GSE 4332) included expression profiles of HSCs from aging C57GL/6 mice (3 arrays for younger: 2 – 3 months and 5 arrays from older: 22 – 24 months).16 The C57GL/6 HSCs represented a highly enriched long-term repopulating (LTR) population as defined by multilineage reconstitution in murine transplant models and the KLSflk-CD34- phenotype.16 The Chambers et al. data set (GEO, GEO accession: GSE 6503) included expression profiles of hematopoietic stem/progenitor cells from C57B1/6 mice at varying ages (2, 6, 12, and 21 months). 17 Two arrays at each age were available.17 The C57GL/6 hematopoietic stem/progenitor cells were selected based upon side population (SPlow), c-Kitpos, lineageneg, and Sca-1pos (e.g. SParKLS).17 gcRMA was used to normalize the murine CEL file data and to compute log2 expression values.6,11,12 Because different Affymetrix murine array platforms were used with the murine studies (Rossi, 430-2.0 and Chambers, MOE430A), normalization and analyses were performed independently for each study. Log2 expression values were analyzed in GenePlus software (Enodar Biologic). For the Rossi data, a two group comparison (young vs. old) was performed. In the case of the Chamber data, data at 4 different ages were available; thus, age was examined as a continuous variable. As with the human analyses, Z-values 4.75 or ≤ -4.75 were consider to be statistically significant.14,15 Of the 161 significant age-associated genes from the human BMCD34+ data set, homologous murine probe sets were available for 144 and 130 genes on the murine arrays used by Rossi and Chambers, respectively. Of the 918 significant genes from the human PBCD34+38- data set, homologous murine probe sets were available for 651 and 570 genes on the murine arrays used by Rossi and Chambers, respectively. Network generation and pathway analyses Data sets of statistically significant genes, including Affymetrix probe ID and corresponding Z-score, were loaded into Ingenuity Pathways Analysis (IPA) software (Ingenuity Systems, www.ingenuity.com) and analyzed.6,18,19 The significance of functional pathways was measured by IPA based on righttailed Fisher Exact Test as previously described.18,19 Functional pathways with a P-value ≤ 0.05 were considered to be statistically significant. Quantitative RT/PCR IRF8 assays in PBSC CD34 and T-cells Quantiative RT/PCR (Q-RT/PCR) assays were primarily performed using samples that had not been previously examined by arrays (Supplement 2). However, the limited number of BMCD34+ and PBCD34+ samples required that some of the samples previously examined by arrays also be used in the QRT/PCR studies. Four of the six BMCD34+ samples in the Q-RT/PCR studies had previously been examined using microarrays, while one of the five PBCD34+ samples in the Q-RT/PCR studies had previously been sorted into PBCD34+38subpopulations and used for arrays. None of the T-cells (N = 26) or PBCD34+38- samples in Q-RT/PCR studies had previously been examined in array analyses. Q-RT/PCR assays were performed using universal TaqMan conditions and the ABI PRIZM 7900HT Sequence Detection System (Applied Biosystems, Foster City, CA).6,20 RT was performed using 0.5 µg of total RNA, AMVRT (Invitrogen, Carlsbad, CA), and Oligo dT primer (Invitrogen) as per manufacturer’s protocol. The cDNA equivalent of 0.05 µg of starting total RNA was used as template for Q-PCR. Each reaction included 2x TaqMan® Universal PCR Master Mix without uraci-N-glycosylase (Applied Biosystems), 800 µM of Reverse and Forward primers, 200 µM of probe, and enough water to bring the total volume up to 25 µL. Sequence specific primers and probe for both IRF8 are as follows: IRF8F (forward): 5’-GAGAGCTGCAGCAGTTCTATAACA-3’; IRF8R (reverse): 5’GCCAGTTGCCGGACATACAG-3’; and Probe: 5’-FAM-CCAAACTCATTCTCGTGCAGATTGAGCAG-TAMRA-3. Primers were shown to amplify the gene of interest by sequencing without aberrant amplification of DNA or other transcripts. Plasmids containing the amplicons for IRF8 and beta-2 microglobulin (B2M) were cloned into pCR2.1TOPO vectors (Invitrogen), and isolated using Plasmid Midi-prep Kit (Qiagen, Valencia, CA). These plasmids were quantified and used to develop standard dilution curves. The copy numbers for IRF8 and B2M were determined based on cycle thresholds (Cts), which were extrapolated to the Cts of standard curves as previously described.20-23 IRF8 copy numbers were adjusted by B2M copies to correct for possible variations in RNA integrity, eliminating samples with poor RNA quality (B2M ≤ 105 copies/50ng).20,22,23 All experiments had a negative control (reaction without a template) to assess for contamination. Linear regression analyses were used to determine the association between age and IRF8 expression when age was treated as a continuous variable. Student’s t-test with two-tail distribution and two-sample unequal variance was used to determine statistical significance between two groups. Western blots Protein lysates were obtained from PBCD34+ cells and quantified as previously described.21 Twenty micrograms of protein combined with 4x buffer (Invitrogen), boiled 5 minutes, loaded into precast 4 – 12% BisTris Gels (Invitrogen), electrophoresed and then transferred to a Hybond-P PVDF membrane (GE Healthcare, Piscataway, NJ) with a mini trans-blot cell (BioRad, Hercules, CA) using a transfer buffer of 25 mM Tris, 192 mM glycine, and 20% methanol.21 The membranes were blocked overnight with 5% nonfat dry milk in ddH2O, washed with TBST x times, and incubated for 1 hour at room temperature (RT) in ddH2O with 5% milk containing polyclonal goat anti-IRF8 at 1:100 dilution (SC-6058, Santa Cruz Inc., Santa Cruz, CA). The blot membrane was then washed with TBST 0.1% 5 times, and reprobed with donkey anti-goat secondary antibody at 1:2500 dilution (SC-2020, Santa Cruz). The membrane was washed 6 times and immunological complexes were visualized using enhanced chemiluminescence (RPN2106, GE Healthcare). Blot membranes were washed overnight in TBST 0.1%, blocked 1 hour at RT in ddH2O with 5% milk, and then incubated with polyclonal rabbit anti-GAPDH (ab-9485-100, Abcam, Cambridge, MA) for 1 hour in ddH2O with 5% milk. The blot membrane was then washed with TBST 0.1% 5 times, and reprobed with donkey ant-rabbit antibody (NA934V, GE, Healthcare) at 1:2500 for 1 hour in ddH2O with 5% milk. The membrane was washed 6 times and immunological complexes were visualized using enhanced chemiluminescence (RPN2106, GE Healthcare). The image was scanned into ImageJ, inverted, and background subtracted using a roller ball radius 20.24 The integrated density for IRF8 and GAPDH were calculated 3 times for each sample, and the average of the 3 calculations used for comparison analyses.24 IRF8 signal was adjusted to GAPDH signal by multiplying the IRF8 signal of the sample by the GAPDH signal for sample divided by median GAPDH signal [IRF8s x (GAPDHm/GAPDHs)]. Supplementary References 1. Phelan EA, Anderson LA, LaCroix AZ, Larson EB. Older adults' views of "successful aging"--how do they compare with researchers' definitions? J Am Geriatr Soc. 2004;52:211-216. 2. Colter M, Jones M, Heimfeld S. CD34+ progenitor cell selection: clinical transplantation, tumor cell purging, gene therapy, ex vivo expansion, and cord blood processing. J Hematother. 1996;5:179-184. 3. Yu J, Leisenring W, Fritschle W, et al. Enumeration of HPC in mobilized peripheral blood with the Sysmex SE9500 predicts final CD34+ cell yield in the apheresis collection. Bone Marrow Transplant. 2000;25:1157-1164. 4. Pittner BT, Shanafelt TD, Kay NE, Jelinek DF. CD38 expression levels in chronic lymphocytic leukemia B cells are associated with activation marker expression and differential responses to interferon stimulation. Leukemia. 2005;19:2264-2272. 5. Levine BL, Mosca JD, Riley JL, et al. Antiviral effect and ex vivo CD4+ T cell proliferation in HIV-positive patients as a result of CD28 costimulation. Science. 1996;272:1939-1943. 6. Stirewalt DL, Meshinchi S, Kopecky KJ, et al. Identification of genes with abnormal expression changes in acute myeloid leukemia. Genes Chromosomes Cancer. 2007. 7. Stirewalt DL, Pogosova-Agadjanyan EL, Khalid N, et al. Single-stranded linear amplification protocol results in reproducible and reliable microarray data from nanogram amounts of starting RNA. Genomics. 2004;83:321-331. 8. Affymetrix I. GeneChip® Expression Analysis Technical Manual. Vol. 2003: Affymetrix, Inc.; 2003. http://www.affymetrix.com/support/technical/manual/expression_manual.affx. Accessed January 25, 2008. 9. Affymetrix I. GeneChip“ Expression Analysis: Data Analysis Fundamentals. Vol. 2003 (ed 701190 Rev. 3): Affymetrix, Inc.; 2003. http://www.affymetrix.com/support/downloads/manuals/data_analysis_fundament als_manual.pdf. Accessed January 25, 2008. 10. Brazma A, Hingamp P, Quackenbush J, et al. Minimum information about a microarray experiment (MIAME)-toward standards for microarray data. Nat Genet. 2001;29:365-371. 11. Bolstad BM, Irizarry RA, Astrand M, Speed TP. A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics. 2003;19:185-193. 12. Irizarry RA, Hobbs B, Collin F, et al. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics. 2003;4:249-264. 13. Stirewalt DL, Clurman B, Appelbaum FR, Willman CL, Radich JP. p73 mutations and expression in adult de novo acute myelogenous leukemia. Leukemia. 1999;13:985-990. 14. Storey JD, Tibshirani R. Statistical significance for genomewide studies. Proc Natl Acad Sci U S A. 2003;100:9440-9445. 15. Xu XL, Olson JM, Zhao LP. A regression-based method to identify differentially expressed genes in microarray time course studies and its application in an inducible Huntington's disease transgenic model. Hum Mol Genet. 2002;11:1977-1985. 16. Rossi DJ, Bryder D, Zahn JM, et al. Cell intrinsic alterations underlie hematopoietic stem cell aging. Proc Natl Acad Sci U S A. 2005;102:9194-9199. 17. Chambers SM, Shaw CA, Gatza C, Fisk CJ, Donehower LA, Goodell MA. Aging hematopoietic stem cells decline in function and exhibit epigenetic dysregulation. PLoS Biol. 2007;5:e201. 18. Ingenuity S. FAQs about statistical considerations. Vol. 2006; 2006. https://analysis.ingenuity.com/pa/info/help/faqs_about_statistical_calculations.ht m. Accessed January 25, 2008 19. Ingenuity S. Guidelines for publications. Vol. 2006; 2006. https://analysis.ingenuity.com/pa/info/help/guidelines_for_publications.htm. Accessed January 25, 2008. 20. Tse W, Meshinchi S, Alonzo TA, et al. Elevated expression of the AF1q gene, an MLL fusion partner, is an independent adverse prognostic factor in pediatric acute myeloid leukemia. Blood. 2004;104:3058-3063. 21. Stirewalt DL, Appelbaum FR, Willman CL, Zager RA, Banker DE. Mevastatin can increase toxicity in primary AMLs exposed to standard therapeutic agents, but statin efficacy is not simply associated with ras hotspot mutations or overexpression. Leukemia Res. 2003;27:133-145. 22. Stirewalt DL, Guthrie KA, Beppu L, et al. Predictors of relapse and overall survival in Philadelphia chromosome-positive acute lymphoblastic leukemia after transplantation. Biol Blood Marrow Transplant. 2003;9:206-212. 23. Stirewalt DL, Willman CL, Radich JP. Quantitative, real-time polymerase chain reactions for FLT3 internal tandem duplications are highly sensitive and specific. Leukemia Res. 2001;25:1085-1088. 24. Rasband WS. ImageJ. U S National Institutes of Health, Bethesda, Maryland, USA. 1997 - 2007;http://rsb.info.nih.gov/ij/. Accessed December 3. 2007.