RCM_4694_sm_SuppInfo
advertisement

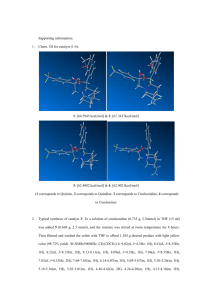
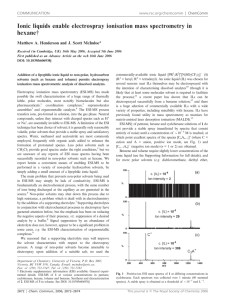
SUPPORTING INFORMATION Gas-phase synthesis of hydrodiphenylcyclopropenylium via nonclassical Favorskii rearrangement from alkali-cationized α,α'-dibromodibenzyl ketone Zhi-Xiong Zhao, Hao-Yang Wang*, Chu Xu and Yin-Long Guo* Shanghai Mass Spectrometry Center, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai 200032, P.R. China *Correspondence to: Y.-L. Guo or H.-Y. Wang, Shanghai Mass Spectrometry Center, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai 200032, P.R. China. E-mail: ylguo@mail.sioc.ac.cn, haoyangwang@mail.sioc.ac.cn 1 1. Synthesis of α,α’-dibromodibenzyl ketone 1 and diphenylcyclopropenone 2 A. Synthesis of α,α’-dibromodibenzyl ketone 1 from dibenzyl ketone To a solution of 7 g (33 mmol) of commercial dibenzyl ketone in 25 mL of glacial acetic acid, a solution of 11 g (67 mmol) of bromine in 50 mL of acetic acid was added with stirring over 15 min at ambient temperature. After the addition was complete, the mixture was stirred until the color of the solution turned to orange-yellow (heat when necessary). The solution was then poured into 100 mL of water. Solid Na2SO3 was added in small portions until the initial yellow color of the solution was discharged, and the mixture was allowed to stand for 1 h. The slightly yellow mixture of meso- and dl-α,α'-dibromodibenzyl ketone was collected and air dried. Recrystallization from hexane (ca. 100 mL) afforded 9.3 g (25mmol, 79%) of white needles. Found: 1H-NMR (300MHz, δ, CDCl3): 7.4 (10H), 5.7 (2H). EI-MS: m/z 368. B. Synthesis of diphenylcyclopropenone 2 from α,α’-dibromodibenzyl ketone 1 This mixture of isomers (7.2 g, 19.6mmol) was dissolved in 33 mL of methylene chloride and the solution was added with stirring over 1 h to 7.2 mL of triethylamine in 16.5 mL of methylene chloride at room temperature. The mixture was stirred for an additional 30 min, extracted with 12 mL 3 N HC1 three times (discarded), and the organic phase was transferred to a flask and cooled in an ice bath. A cooled solution of 6 mL of concentrated H2SO4 in 2 mL of water was slowly added into the organic phase. A slightly pink precipitate of diphenylcyclopropenone bisulfate separated and this was collected on a sintered glass funnel. The solid was then returned to the flask along with 18 mL of methylene chloride and 36 mL of water, and 0.35 g of solid Na2CO3 was added in small portions. The organic layer was collected and the aqueous solution was extracted with three 18 mL portions of methylene chloride. The combined organic layers were dried over MgSO4 and evaporated to dryness. The 2 impure diphenylcyclopropenone was recrystallized by repeated extractions with boiling cyclohexane (total 100 mL), the solution being decanted from a reddish, oily impurity. On cooling, the solution deposited white crystals 1.8 g (9.7 mmol, 45%) of diphenylcyclopropenone. Found: 1H-NMR (300MHz, δ, CDCl3): 8.0 (4H), 7.6 (6H). EI-MS: m/z 178 (as the standard mass spectrum). Figure S1. 1H-NMR spectrum for α,α’-dibromodibenzyl ketone. 3 Figure S2. 1H-NMR spectrum for diphenylcyclopropenone. 4 2. Characterization of α,α’-dibromodibenzyl ketone 1 ESI mass spectra of α,α’-dibromodibenzyl ketone 1 Figure S3. ESI-MS spectrum of α,α’-dibromodibenzyl ketone 1 in methanol. Figure S4. ESI-MS spectra of α,α'-dibromodibenzyl ketone 1 in methanol with alkali-metal salt: (a) LiCl and (b) NaCl. 5 ESI-MS/MS of 1·Li+ Figure S5. ESI-MS/MS spectra for CID of 1·Li+: (a) at m/z 373; (b) at m/z 375; and (c) at m/z 377 (CID energy at 20 eV by TSQ). The product ion for 2·H+ at m/z 207 could also be observed in the ESI-MS/MS spectra of 1·Li+ and the ESI-MS/MS spectra of 1·Na+. However, a 'byproduct' ion at m/z 214 could also be observed clearly. The reason for the formation of this ion was attributed to the behavior of the Li atom. As a stronger Lewis acid than Na, Li shows stronger affinity to oxygen. Furthermore, the atomic radius of Li+ is smaller than that of Na+. For these reasons, the interaction between the Li+ and the bromide atom in the five-membered ring mentioned in the full text might be weaker than that of Na+ and the catalytic debromination effect was also weaker for Li+. Thus, we proposed the 'side reaction' fragmentation pathway shown in Scheme S1. The proposed structure for the ion at m/z 214 formed by loss of [H+2Br] from 1·Li+ seem to more stable for the aromatic effect. 6 Scheme S1. The proposed dissociation pathway of the 1·Li+ ion at m/z 373. 7 3. Characterization of diphenylcyclopropenone 2 Standard EI-MS (70 eV) of diphenylcyclopropenone 2 Figure S6. Standard EI-MS spectrum of diphenylcyclopropenone 1 (data from NIST 08 Mass Spectral Library). ESI mass spectra of diphenylcyclopropenone 2 Figure S7. ESI-MS spectrum of diphenylcyclopropenone 1 in methanol. 8 Figure S8. ESI-MS/MS spectrum of the fragment ion at m/z 179 (CID energy at 10 eV by TSQ). 9