lab VII
advertisement

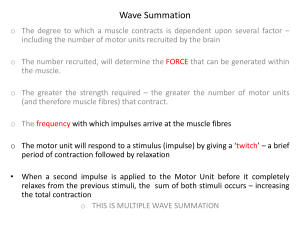
LAB VII Muscle Function II: Muscle Performance, Mechanisms of Fatigue, and Neural Control of Muscle Last week we focused on learning about use of electromyography to study muscle function and on factors that influence muscular force production. For example, we discussed how motor unit recruitment, joint angle, type of action, and velocity influence muscular force production. This week we will discuss a few more variables that can influence muscular force production under certain circumstances: fatigue and use of the stretch shortening cycle. While force production may be very important for some individuals and specific types of athletes, not all daily living activities and athletic events rely on force production alone. The force that can be produced would certainly influence performance in sports where strength is required. In other sports, force may be important (such as in throwing a shot put) but require that the force be produced rapidly (with high velocity). As discussed earlier in the quarter, Power is equal to force times velocity. Thus, power is of great importance for some athletes while strength or velocity of movement may be more important in others. In this lab we will evaluate the relationship between muscular force, velocity, and power. Not all athletic endeavors require high velocities or high forces, but require sustained effort. Such events are dependent upon the ability to resist fatigue; that is, endurance. In several labs this quarter we have been (and/or will be) concerned with endurance performance during whole body exercise (e.g. cycling, running) and we discussed the roles of VO2max, lactate threshold, economy of movement, and fuel substrate use that may influence the ability to perform well in distance races. In this lab we will be more concerned with muscular endurance; endurance during isolated muscle group exercise. The ability to resist fatigue (endurance), force (strength), velocity (speed), and power (power) constitue our different muscle performance attributes. Our final topic for this lab has also been partly covered before: neural control of muscle. We have already discussed motor unit recruitment and how we can evaluate it using EMG. That is, we have been focusing on studying the end result of the neural stimulation of motor units. But motor control is a lot more complicated than just the motor output. The signals that resulted in action potentials in our motor neurons originate, in voluntary movements, further up in the central nervous system, in motor regions of the cerebral cortex. Additionally, the nervous system is constantly taking in information from a variety of sources; especially from our proprioreceptors. This sensory information is taken in, and compared to our planned movements so that we can adjust our motor unit recruitment pattern accordingly. Additionally, there are a number of applications of using these proprioreceptors. For example in this lab we will study the potential use of proprioreceptors in allowing us to jump higher when making use of the stretch-shortening cycle and we will also study how use of proprioreceptors may also allow us to gain greater range of motion and improving flexibility. Muscular Force Production Review and Muscle Performance Preview The most common measure of dynamic strength is the 1RM or 1 repetition maximum. The 1RM is the maximal resistance (weight) that can be moved through a designated range of motion a single time. The procedures for determining a subject’s 1RM can be found in the appendix (appendix p. 4). Typically the subject will perform two to three warm-up sets before their first attempt at 1RM. If the target attempt is made the resistance is increased with each subsequent attempt, resting in between attempts, until the subject fails to lift the weight. The last successful lift is the 1RM. It is possible to make evaluations of strength during both the concentric and eccentric phases of dynamic actions. Lab VII - 1 Endurance can be assessed by determining how long (a time) the subject can sustain some percentage of their maximal force (can use isometric or dynamic tests). Power cannot be evaluating during isometric actions because there is no movement and therefore no velocity. Dynamic muscular endurance can be evaluated by having your subject perform as many repetitions as possible at a certain percent of their 1RM effort. Muscular power can be assessed if you know the following: the resistance, the distance moved against gravity, and the time it took to move this distance. Power can then be calculated (as we did in lab 1): power = force x distance / time (or power = force x velocity). The effect of movement velocity on the ability of muscle to produce force, makes speed a factor that you may want to control during the evaluation of force production. This can be accomplished using an isokinetic device. As discussed in the previous lab, these devices allow muscular activities to be performed at a constant angular velocity (joint speed) with an accommodating resistance. One benefit of using these devices for strength tests is that they allow the clinician or researcher to control the velocity of movement. Thus, these devices allow clinicians and researchers to control for the effect of velocity on muscular force production. The stretch-shortening cycle or pre-loading of the muscle with an eccentric stretch has been reported to produce greater force production in the following concentric action. This phenomena may be the result of stored elastic energy during the pre-stretch (stretching the elastic components of the muscle) or by increasing MU recruitment via a stretch reflex. There are many possible contributors to the elastic properties of muscle (and thus to passive tension). One of the most important elastic components of muscle is the protein titin, which extends between the thick filaments and the z-lines. Additionally, collagen and elastin found in the tendons and muscle connective tissue (epimysium, perimysium, and endomysium) may also contribute. Fatigue and Muscular Performance Several definitions of fatigue exist in the scientific literature. For example, some define fatigue as a decline in the maximal force that can be generated by a muscle; others define it as an inability to sustain some submaximal exercise intensity. A broad, but appropriate, definition for this course is that fatigue is an inability to maintain a desired level of performance. For most individuals, maximal contractions are somewhat uncommon in our daily lives, and when they occur, they are rarely sustained for more than a few seconds. Thus, defining fatigue based on a reduced maximal force production would not adequately describe the type of fatigue one encounters on a regular basis. On the other hand, defining fatigue as an inability to maintain a desired level of performance adequately describes the reduction in performance when submaximal exercise is performed over many seconds, minutes, or hours. It is worth noting that the terms fatigue and muscle fatigue are some times used interchangeably. Fatigue is reportedly the most common presenting symptom in the primary health care setting and there are many disease states that result in noteworthy fatigue. Fatigue is also an important determinant of endurance performance and may protect muscle cells from irreversible damage. Fatigue is inversely proportional to endurance performance; the greater the severity of fatigue (or the sooner in exercise that it occurs), the poorer the endurance performance. Individuals who wish to improve endurance performance generally do so by mechanisms that delay the onset of fatigue. Furthermore, premature fatigue during training (any type of exercise training: endurance, power, or strength) will cut short effective training time. Many of the changes that occur during the fatigue process could be damaging (or even lethal) for the muscle fibers. Thus, it is believed by some that fatigue can be thought of as a protective mechanism. For example, if the ATP concentration ([ATP]) were to fall too low, the cell would not survive. The [ATP] in the cell is dependent upon both how quickly it is being produced and how quickly it is being used. There is evidence that anaerobic and aerobic metabolism can not produce Lab VII - 2 ATP as quickly when the muscle fibers are fatigued. By decreasing the muscles' force (or power) output during fatiguing contractions, the rate of ATP utilization is reduced. This decrease in ATP utilization will prevent a lethal drop in cellular [ATP], and thus allow the cells to survive. In deed, skeletal muscle cells appear to be very protective of their intracellular [ATP]. The average [ATP] in mixed and slow twitch fibers generally do not fall below 70% of their resting concentration, even during severe fatiguing contractions. In IIX fibers on the other hand the rate of ATP use is so fast that these fibers may have a drop in [ATP] as low as 20% of resting values. However, these are not sustainable. Considerable progress has been made in understanding the physiological causes and consequences of muscular fatigue. Despite this progress there is still considerable disagreement in the scientific literature as to the specific causes of fatigue and the sites where it originates. This is truly amazing when you consider that exercise-induced muscular fatigue has been studied for over a century! Part of the reason for this disagreement is that muscle fatigue studies have used different modes of exercise, different intensities, different durations, different rest periods, different organisms, different muscle groups, and different environments. Some studies have used voluntary contractions, others have used electrical stimulation, and some have used a combination of stimulation and voluntary activity. It is important to keep in mind that the causes of fatigue can be very different under different exercise conditions. For example, during a sustained maximal voluntary contraction (MVC, a maximum isometric contraction performed voluntarily by the subject) the causes of fatigue appear to be quite different from the causes of fatigue during sustained submaximal contractions. Additionally, most studies on muscle fatigue have focused on isometric activities with small, isolated muscle groups. The results of such studies are, in many cases, not directly applicable to whole body exercise under competitive conditions. Another difficulty in studying fatigue is that fatigue-related processes appear to start early during a contraction, with the symptoms occurring later. Fatigue is a multi-factorial process. Thus, there may be no single cause or site of fatigue, even in highly specific and controlled situations. A solid understanding of fatigue, and fatigue related processes, are very important for those who work in rehabilitation and for those whose job is to help an athlete reach their full potential. Because there are so many possible causes and consequences of fatigue, which may differ from one activity to another, it is important to understand as many of these processes as possible. It is sometimes helpful to generalize the sites causing fatigue as either central or peripheral (see figure 1). In general, central fatigue refers to fatigue that is due to inadequate nervous system activation of the muscle fibers (could be in the central or peripheral nervous system. On the other hand, peripheral fatigue refers to causes of fatigue within the muscle fiber(s). One way to do this is to have the subject perform muscular activity to fatigue and assess the subject's maximum ability to produce force voluntarily (MVC) and maximum force that can be produced using an electrical stimulus that is strong enough to maximally activate the muscle. The maximum force that can be produced during such electrical stimulation is called the maximum evocable force (MEF). If the site of fatigue is central (there is inadequate voluntary neural activation of the muscle) then more force will be produced with the strong electrical stimulation of the muscle (MEF) than during a maximal voluntary contraction (MVC). That is, the decrease in maximum voluntary contraction force is caused by a reduced ability of the nervous system to stimulate the muscle. Åstrand and his co-authors define central fatigue as an exercise-induced reduction in MVC force while MEF is maintained. If the fatigue is peripheral in origin, then electrical stimulation will not increase force production above the maximum voluntary contraction force. Åstrand and his co-authors define peripheral fatigue as a decrease in maximal force production even when the motor neurons are stimulating the muscle fibers optimally. This means that the muscle fibers are not contracting maximally even though there is adequate stimulation; the site causing the fatigue is in the muscle itself. Lab VII - 3 As you can see in figure 1, the delineation of central versus peripheral fatigue does little in pinpointing the actual "cause" of fatigue. EMG can be used to help identify the site of fatigue. In this lab we will use EMG to try and determine if the source, or cause, of fatigue lies within the nervous system or within the muscle. If a subject experiences central fatigue, then you would expect to observe lower iEMG integrals (less EMG activity) coinciding with the reduced performance. If the site of fatigue is peripheral, then as muscular performance is compromised, the EMG activity should remain the same (or even increase). This is not a clear-cut matter. For example, a decrease in neural activation of the muscle could result from afferent input (sensory information, represented by dashed lines in figure 1) originating in the fatigued muscle fibers. That is, afferent information coming into the central nervous system may inhibit, via an inhibitory interneuron, the output of the motor neurons. In this scenario, there would be a decrease in EMG activity, but the cause of fatigue would actually be in the muscle! An additional problem in using EMG to assess fatigue, is that it is difficult in most cases to obtain EMG from every muscle involved in a particular exercise. For example, even a simple biceps curl uses at least three muscles; the biceps brachii, the brachioradialis, and the brachialis, of which EMG can only be obtained from the biceps and the brachioradialis. A. Higher Motor Centers Of the Brain B. Spinal Cord I. Central Fatigue C. Peripheral neurons Muscle fiber A. Sarcolemma B. T-Tubules C. Sarcoplasmic Reticulum (Calcium release/uptake) D. Energy Pathways II. Peripheral Fatigue E. Actin-myosin interactions & cross-bridge cycling Force and Power Output Figure 1. Possible sites of central and peripheral fatigue Lactic acid is one of the products formed as a result of anaerobic glycolysis. Like most acids, lactic acid readily dissociates from one of its hydrogen ions under physiological conditions (conditions normally found in the body). Thus, it may be more appropriate to refer to lactic acid by the name of its salt; lactate. Many physiologists use the terms lactate and lactic acid interchangeably. It is worth noting that the term anaerobic in the phrase anaerobic glycolysis refers to the fact that this pathway does not require oxygen (it does not actually mean that the cell is lacking oxygen). It has been demonstrated that muscles (and other tissues) use anaerobic glycolysis when the cells' rate of ATP use is greater than the rate at which aerobic metabolism can create ATP, even if there is sufficient oxygen. Lab VII - 4 It is therefore important that students avoid thinking of lactate as something that is formed only when a cell lacks oxygen. It is also important to note that lactate is not just a by-product of anaerobic metabolism; it can play some important roles in the body. Additionally, it is important to understand that the amount of lactic acid inside a muscle fiber is dependent on both the rate of its production and on the rate of its clearance (removal) from the fiber. Likewise, the amount of lactic acid in the circulation is also dependent upon the balance of production and clearance. It was thought for a very long time that lactic acid caused fatigue. This was based on the fact that high muscle and blood lactate concentrations were associated with fatigue. However, it is now understood that lactate itself does not cause fatigue. Instead, the hydrogen ions (H+) that dissociate from lactic acid that may cause fatigue by several possible mechanisms. Furthermore, there are several other changes (e.g. increases in inorganic phosphate and ADP) that occur in the muscle during fatigue and it is not clear whether these H+ are causing the fatigue or if something else is causing fatigue. Just because muscle pH is low when the muscle fatigues does not mean that low muscle pH is causing fatigue. It is important to note that all muscle fibers are not equally prone to fatigue. Oxidative fibers have greater fatigue resistance than fast glycolytic fibers (see lab 2 for details and re-read if necessary). The following section is a relatively short list of potential causes of fatigue and explanations of how these fatigue mechanisms might act to affect muscle performance. The outline letters and numbers refer to their location on figure 1. It is also important for you to note which sites are likely to be causes of fatigue under conditions observed in our lab. I. Central fatigue If central fatigue occurs, one would observe a decrease in MVC force, but not the maximum evocable force. Additionally, if the cause of the fatigue is central (neural) then one should observe a decrease in EMG activity concomitant with the reduced performance. This could be a result of reduced neural drive in the central nervous system, from changes at the level of the motor neuron itself, or from changes in afferent input to the motor neurons (part of the peripheral nervous system). For example, it is clear that motor neuron firing rate decreases during many types of fatigue, such as during maximal voluntary contractions. A & B. Brain & Spinal Cord (central nervous system). There are observable changes in the motor cortex during fatiguing contractions. However, there is still considerable controversy about whether or not these changes cause muscle fatigue or simply coincide with it. For example, high concentrations of serotonin and/or low dopamine levels (or reduced dopamine signaling) in certain parts of the brain have been associated with fatigue. Such may explain some of the fatigue related symptoms in depression and chronic fatigue syndrome. The central nervous system would also likely be the site responsible for fatigue resulting from poor motivation, although this is more of a psychological than physiological fatigue mechanism. When subjects are experiencing central fatigue, it is noteworthy that the subject feels that the effort is becoming increasingly challenging to maintain. It appears that central mechanisms of fatigue in cortical regions (and influenced by afferent nerves from skeletal muscle) may play a significant role during sustained maximal contractions. Another scenario under which the central nervous system plays an important role is when the brain/core temperature exceeds 40C (hyperthermia). C. Peripheral nervous system (motor neurons & afferent neurons involved in motor circuits). The excitability and firing rate of the motor neurons can be affected by a number of mechanisms. In general, a decrease in motor neuron excitability or firing rate could result from a decrease in excitatory impulses reaching the motor neuron or from an increase in inhibitory impulses reaching the motor neuron, or both. A decrease in excitatory impulses reaching the motor Lab VII - 5 neurons could be due to a decrease in excitatory information coming down the spinal cord from motor centers of the brain (see CNS above), or to a decrease in excitatory afferent information coming from the muscle spindles (if the muscle becomes less active then the muscle spindles will become less active). An increase in inhibitory impulses, mediated by inhibitory interneurons, could be caused by an increase in afferent activity from (group III & IV) nerve endings that detect chemical changes (especially hydrogen ions) in the muscle fibers. A decrease in motor neuron firing rate has been consistently demonstrated during fatiguing maximal isometric contractions. While it may seem that this could be detrimental to performance, it may actually be beneficial. As muscle fibers fatigue, the contraction and relaxation times of their twitches increase. This increases the duration of each twitch and thus decreases the firing rate required to reach tetanus. This phenomenon has been called muscle wisdom. It appears that afferent nerve endings (such as those that detect chemical changes) in the active muscles act to feed information back to the motor neurons. This allows the motor neuron firing rate to be matched to the contractile properties of the muscle fibers, and thus may help prevent fatigue. While this phenomenon has been well documented during maximal isometric actions with small muscle groups, it does not consistently occur during submaximal and dynamic conditions. The ability of a motor neuron to stimulate the muscle fiber is also dependent upon events at the neuromuscular junction (NMJ) where the motor neurons innervate the muscle fibers. It is possible that under certain situations that an impulse reaching the NMJ could fail to elicit a response in the muscle fiber (e.g. if too little acetylcholine is released). Most, but not all, studies demonstrating this type of fatigue have used electrical stimulation frequencies well above those that normally occur in the body. II. Peripheral fatigue – Most available evidence suggests that the causes of muscle fatigue observed under normal physiological conditions are distal to the neuromuscular junction (within the muscle fibers themselves). In peripheral fatigue, maximal electrical stimulation will fail to increase force production above that which can be produced voluntarily. Additionally, EMG activity would be expected to stay the same, or even increase, if the cause of fatigue is within the fibers themselves. A & B. Sarcolemma & T-tubules. During fatiguing muscle contractions it is clear that potassium (K+) accumulates outside the sarcolemma. This accumulation of potassium has been associated with a slight depolarization of the membrane at rest and a longer and smaller action potential. A chronic, low level depolarization of the membrane would prevent Na+ channels from resetting themselves following an action potential (when they close they become temporarily inactivated). Some physiologists believe that these problems would be even worse in the T-tubules than at the sarcolemma. It is therefore possible that in fatigued muscles, the action potentials may fail to spread down the sarcolemma and down the T-tubules. If this is the case, calcium would not be effectively released in the effected fibers. Fortunately we have several mechanisms that help to maintain membrane potential in the face of these unfavorable changes in [K] in and out fo the cell. C. Sarcoplasmic reticulum (SR; calcium release and uptake). Fatigued muscle fibers to not appear to release as much calcium when stimulated compared to unfatigued fibers, even if the membrane properties are unaffected. Experimental evidence suggests that changes at the level of the SR calcium release channels (ryanodine receptors) are may be partly responsible for the reduced calcium release. This may be related to reduced [ATP], elevated Mg2+, and or elevated inorganic phosphate (Pi). Pi may play an additional role. As ATP is rapidly degraded, there is a large increase in [Pi], some of which leaks into the SR and precipitates with Ca2+. This is believed to be a major contributor to fatigue. Lab VII - 6 Calcium uptake by the SR calcium ATPases (SERCAs) also appears to be impaired in fatigued muscle fibers. It is this reduced uptake of calcium back into the SR that prolongs the relaxation time. This slowing of SERCA activity is likely a result of any, some, or all of the following: elevated [Pi], elevated [ADP], lower [ATP], and/or perhaps an increase in reactive oxygen species. Slowing of calcium reuptake and subsequent slowing of relaxation could play a role during repetitive movements. It is noteworthy that calcium initiates contractile processes (which increase ATP use) and that a reduction in calcium release may be responsible for slowing us down/reducing our force when we are fatigued (which would decrease our ATP use). The reduced calcium release during fatigue would slow ATP use and thus help protect cellular ATP levels. However, the protection of [ATP] in this case would be at the expense of muscle performance (must slow down and/or reduce force). D. Energy pathways 1. Phosphocreatine (PCr). PCr is a high energy phosphate that plays an important role in maintaining and protecting cellular ATP concentrations: PCr + ADP ATP + Cr This is the muscle cell's most rapid mechanism of producing ATP. During exercise PCr is degraded (hydrolyzed) rapidly to form ATP (and creatine). During recovery after exercise ATP production remains elevated for a time to help the cell return to normal, including replenishing PCr stores. This reversible reaction is catalyzed by the enzyme creatine kinase. The higher the intensity, the faster PCr is broken down. A large body of evidence suggests the PCr depletion is a major contributor to fatigue during short term, high intensity exercise. Some athletes take creatine, which is normally found in meats, as a supplement in the hopes that it will increase PCr stores in the muscle. There is a large body of experimental evidence suggesting that creatine supplementation improves some types of exercise performance and in certain types of individuals. However, there is also a large body of evidence suggesting that this expensive supplement does not improve performance. 2. Adenosine triphosphate (ATP). ATP is the immediate source of energy for contractile processes. It is also necessary for many other cellular processes, including maintenance of the membrane potential. Nearly every change that occurs in the body during exercise can be directly, or indirectly, linked with the increased energy (and thus ATP) demand of the active muscles. It was once thought that an inability to produce ATP in sufficient quantities caused fatigue. However, as mentioned earlier, cellular ATP concentrations are well protected (a large drop in cellular ATP would be lethal for the cell). Thus, it is unlikely that a drop in ATP production directly causes fatigue. But, this does not mean that ATP lacks a role in muscle fatigue. It is possible that fatigue occurs in order to protect cellular ATP concentrations. By reducing force production, or power output, the muscle cell's rate of ATP use is reduced, thus preventing a decline in ATP concentrations, even if the rate of ATP production is impaired. There is also evidence that high intracellular ADP concentrations and low intracellular pH (as occurs in fatiguing muscle) reduce the free energy released during ATP hydrolysis. The rate limiting step of the cross bridge cycle is how fast ADP can dissociated from the myosin head. This slows down as [ADP] in the cell increases. This may contribute to reducing the velocity of movement. 3. Metabolite accumulation (hydrogen ions & inorganic phosphate). As discussed previously, lactic acid formation by anaerobic glycolysis can result in an increase in H+ (decrease in pH) in the cell. Many metabolic processes speed up during exercise and contribute to the increase in H+ that occurs during high intensity exercise. These hydrogen ions can cause fatigue through Lab VII - 7 several mechanisms. For example, an increase in H+ can inhibit key metabolic enzymes such as phosphorylase and phosphofructokinase. Phosphorylase is an enzyme that breaks down glycogen and phosphofructokinase is the rate-limiting enzyme in glycolysis. Thus, the decrease in pH in the muscle cells during high intensity exercise can cause both glycogenolysis (breakdown of glycogen) and glycolysis to slow down. H+ may also competitively inhibit the ability of Ca2+ to bind with troponin, thus making it less sensitive to calcium. As PCr concentration decreases during exercise, and ATP is being rapidly broken down the concentration of inorganic phosphate (Pi) increases. There is experimental evidence for each of the following roles or Pi in fatigue processes: reduce Ca2+ sensitivity of myofibrillar proteins, reduce maximal force of myosin heads, precipitation of Pi with Ca2+ in the SR, impair RYR function, and slow SERCA activity. 4. Glycogen & blood glucose. There is a large body of evidence suggesting that during prolonged exercise (greater than 60-90 minutes) fatigue is related to depletion of muscle glycogen stores. This is especially true for marathon runners who describe “hitting the wall” at around mile 20 (earlier for less competitive marathoners), which approximately corresponds with the point in time when glycogen levels reach their lowest levels. Additionally, delaying glycogen depletion appears to delay the onset of fatigue. Interestingly, there is some evidence that low glycogen levels may somehow reduce SR Ca2+ release (via an unknown mechanism). Blood glucose is usually well maintained during exercise. This is understandable since the central nervous system is dependent upon blood glucose as a source of energy. However, during prolonged exercise of sufficient intensity, a drop in blood glucose is possible. If this happens, the CNS is generally depressed, and fatigue ensues. Interestingly, ingestion of a carbohydrate solution during exercise appears to help maintain blood glucose and delay the depletion of glycogen stores. Thus, it is not a bad idea to consume carbohydrate containing fluids during exercise if the individual is going to be performing a long duration exercise bout such as a marathon (26.2 mile run) or a century (100 mile bike race). Consumption of such a fluid would also increase insulin secretion and would thus reduce glycogen use while simultaneously providing another source of carbohydrate for the active muscles. E. Actin-myosin interactions/cross bridges and Calcium Sensitivity. There is good scientific evidence demonstrating that elevated [ADP] can reduce cross bridge cycling, and thus velocity. Also, elevated [Pi] may reduce force produced by individual cross bridges. This appears to be especially true in fast twitch fibers during tetanic contractions. Myofibrillar proteins, such as troponin, are less sensitive to calcium when [Pi] is elevated and pH is low, such as might be the case during fatigue. F. Reduction in blood flow. During sustained isometric actions, pressure in the muscle can exceed arterial pressure, which then reduces blood flow to the contracting muscle. This reduction in blood flow hastens fatigue. Experimental reduction in blood flow using a blood pressure cuff also results in faster development of fatigue. Gender differences in muscle fatigueability may be, in part, related to blood flow during sustained submaximal isometric contractions. At any given percent of maximum contraction, a man’s muscle tends to produce more pressure, and thus a greater reduction in blood flow. The mechanism by which reduced blood flow is not clear but could easily relate not just to delivery of the blood, but also clearing metabolic wastes and by products from the muscle. In summary, fatigue plays an important role in determining endurance performance and may actually serve a protective role for muscle fibers. It is currently unclear to what extent neural factors are involved in the fatigue process during normal muscle activities. If neural factors are involved in the fatigue process, resulting in reduced stimulation of the muscle fibers, one should be able to observe Lab VII - 8 a reduction in EMG activity. However, most evidence suggests that the sites that cause fatigue under normal circumstances lie within the muscle fibers themselves. It is likely that the depletion of PCr stores and accumulation of hydrogen ions and inorganic phosphate contribute to the fatigue process under normal circumstances. Both of these metabolites appear to be able to cause fatigue by several mechanisms. During more prolonged activities depletion of glycogen stores or reduction in blood glucose are associated with fatigue. Fatigue is a complex process that likely involves many of the above mechanisms simultaneously. Neural Control of Muscle and Muscle Reflexes Although it is the muscle that produces the force, the control of movement requires the nervous system. The nervous system functions through two basic divisions: Sensory input and Motor output. When one thinks about movement, it may seem that the motor output side is the most important. However, even during our simplest motor events, reflexes, the sensory input side of the nervous system plays a very important role. Therefore, neuromuscular function requires an integration of sensory input and motor output. During a simple reflex, a stimulus is detected by a specific sensory receptor (remember sensory receptors function according to the laws of: adequate stimulus, specific nerve energies and projection). If the stimulus is of threshold strength, an action potential will occur and travel through afferent nerves to the central nervous system (CNS, remember this includes the brain and spinal cord). In the CNS a decision will be made and if appropriate motor output (response) will be generated. Motor output is the stimulation of motor nerves or motor units. A motor reflex arc is illustrated below. Integrating Center (CNS) Afferent pathway Efferent pathway Sensory receptor Effector Organ Stimulus Sensory Input Motor Output Response The myotatic reflex or stretch reflex is the simplest (monosynaptic) reflex, but is also believed to be important in other more complex neuromuscular events. The stimulus that initiates a myotatic reflex is a sudden stretching of the muscle. The receptor that detects this stretching of the muscle is the muscle spindle. Every skeletal muscle contains tens to hundreds of muscle spindles. Prior to class you must review the anatomical structure of the muscle spindles and their mechanism of action (covered in your human physiology courses). If the spindle stimulus is great enough the motor response will be to stimulate the extrafusal fibers in the muscle receiving the initial stretch. It also should be noted that when extrafusal fibers are stimulated, intrafusal fibers are also stimulated (alpha-gamma coactivation). This reflex keeps the muscle at the same length and prevents sudden undesired changes in body position. This reflex may provide part of the benefit of plyometric activities. The sudden stretch on the muscle during a plyometric activity could elicit a reflex that would be added to the voluntary activation, resulting in greater neuromuscular activation compared to normal muscle actions (i.e. greater than normal motor unit recruitment). Plyometric activities also maximize the use of elastic energy created by stretching the series and parallel elastic components of the muscle. When a muscle spindle is activated, some of the electrical information will also synapse with an inhibitory interneuron in the spinal cord. This inhibitory interneuron, in turn synapses with motor neurons that innervate the antagonistic muscle group. When stimulated, this inhibitory interneuron will release an inhibitory neurotransmitter that will inhibit the formation of an action potential in the motor neurons that innervate the antagonistic muscle group. This reflex inhibition of the antagonistic muscle group is Lab VII - 9 called reciprocal inhibition. Thus, while excitatory information causes stimulation of the muscle where the muscle spindle is activated, the antagonistic muscle group is inhibited. The muscle spindles, in their function as a proprioreceptors also send information to the central nervous system about the length and rate of lengthening of the muscle. Proprioreceptive information, like all sensory information enters the spinal cord via the dorsal root of the spinal cord. Information from muscle spindles and other proprioreceptors, like the golgi tendon organs, send information up the spinal cord via distinct tracts of interneurons, which collectively are referred to as the DorsalLemniscal system. This set of tracts sends information rapidly up the spinal cord. Several other spinal cord tracts transmit information from other types of receptors up to the sensory cortex. Another important reflex in the control of movement is the inverse stretch reflex. The receptor involved in this reflex is the golgi tendon organ (GTO). These receptors can be found in the tendons of skeletal muscle and detect tension or strain on the tendon and muscle. The reflexive response to excessive tension, inhibits the muscle reducing the tension on the tendon. These receptors are believed to function in a protective or injury preventing manner. These receptors are also important proprioreceptors that send information about the amount of tension placed on the muscles to the central nervous system. This information is absolutely necessary so that the central nervous system can determine how many motor units to activate. In addition to proprioreceptors in the muscle and tendon, there are also proprioreceptors in joints, ligaments, and skin. All motor neurons are subject to both inhibitory and excitatory influences. For example, both excitatory and inhibitory neurotransmitters could both be influencing a motor neuron at the same time. Whether or not the motor neuron will have an action potential simply depends on whether or not the membrane potential is brought to threshold or not. If a large amount of excitatory neurotransmitter is present and only a small amount of inhibitory neurotransmitters, then the motor neuron is likely to reach threshold, and therefore lead to stimulation of the entire motor unit. On the other hand if more inhibitory neurotransmitters are present than excitatory then the motor neuron is unlikely to reach threshold. If threshold is not reached, the motor neuron will not have an action potential, and the muscle fibers in its motor unit will not be stimulated. The inverse stretch reflex and the central nervous system’s function of reciprocal inhibition are the basis for some proprioceptive neuromuscular facilitation (PNF) stretching techniques. The holdrelax PNF procedure uses a strong isometric contraction of the muscle to be stretched followed by stretching of the muscle. This strong contraction stimulates the GTO’s in the contracted muscle (antagonist) and produces an inverse stretch reflex (also called autogenic inhibition). It is important to note that the muscle being stretched is the antagonist muscle for that action (e.g. The hamstrings are hip extensors and can be stretched by hip flexion). The inverse stretch reflex causes the contracted muscle (antagonist) to relax and allows a better stretch. The hold-relax with agonist contraction PNF procedure is similar, but following the strong contraction of the muscle being stretched (antagonist), the agonist muscle group contracts. This contraction of the agonist should result in inhibition of the antagonist via reciprocal inhibition, and should thus allow for a greater stretch of the antagonist muscle. The contract-relax PNF procedure is also similar to the hold-relax procedure, but instead of an isometric action, a dynamic action is performed. See page 5 of your appendix to see reflex arcs for reciprocal inhibition and the inverse stretch reflex. Neural Control of Movement: Integration of Sensory Input & Motor Output So far we have discussed a number of properties related to the neural control of skeletal muscle. Wave or twitch summation refers to the increasing firing rate of motor neurons (and thus the muscle fibers that they innervate; i.e. increased firing rates of the motor units) when there is a need to increase force production. We also can increase force production by increasing the number of motor units active at any given time (multiple motor unit summation). We have also demonstrated in previous labs that as we move a limb through its range of motion the motor unit recruitment is not constant. Lab VII - 10 Even with a constant load (e.g. a 5-pound weight) as we move through different joint angles the leverage is constantly changing. The length of the sarcomeres also changes throughout the movement. Thus we must be able to alter the amount of force produced at every moment throughout that movement. We have also briefly discussed the role of proprioreceptors in eliciting various reflexes. Their roles in the control of movement are not confined to just reflexes. It is ultimately information from the proprioreceptors that allows us to change the amount of force produced throughout a joint’s range of motion. The proprioreceptors in the muscle as well as joint kinesthetic receptors are constantly sending information to the CNS about the length of the muscle, the rate of change of muscle length, the amount of tension placed on the muscle, and the joint angle. The CNS then alters the firing rate of the motor units and the number of motor units being fired to create the appropriate amount of tension. Thus, even the simple movement across a single joint requires both sensory input and motor output. However, motor unit recruitment is more complex than just altering the number of motor units being used and the firing rate of those motor units. During sub-maximal contractions the motor units ”take turns” firing and relaxing in order to avoid fatigue. For example consider an isometric action that requires the use of only two motor units at a time, and the motor units being used are named A, B, and C. At first motor units A and B may be used, while motor unit C is relaxing. Then, motor units B and C are fired, allowing motor unit A to relax. Following this, motor units A and C are fired together allowing motor unit B to relax. Then back to A and B firing together and so on. This process of constantly switching the motor units being fired is called asynchronous motor unit recruitment. Keep in mind that this is a highly simplified version of events because most movements require many more than just two motor units at a time. During dynamic muscle actions the CNS controls this asynchronous recruitment of motor units, while at the same time altering the total number of motor units fired, as well as altering the firing rate of the motor units. In this way we can produce smooth movements, altering force production as needed, and at the same time delaying the onset of fatigue. Interestingly, resistance exercise training appears to increase a person’s ability to fire motor units in synchrony during forceful contractions. The end result is that during forceful contractions more motor units can simultaneously be stimulated. An additional consideration when discussing the complexity of even the simplest movements is the order in which motor units are recruited. Smaller motor units tend to have lower thresholds for recruitment. That is, they do not need as great a stimulus to create an action potential. The smallest motor units tend to be composed of type I muscle fibers. Thus, small motor units made up of type I muscle fibers are recruited first because they have the lowest thresholds for recruitment. Type IIX muscle fibers tend to be included in large motor units and Type IIA muscle fibers tend to included in intermediate sized motor units. Because of these differences in motor unit size and threshold for recruitment, motor units with type I muscle fibers tend to be recruited first, no matter how much force is required. With increasing force requirements, more motor units with type I muscle fibers will be recruited. Eventually, as the force requirement becomes greater, motor units with type IIA muscle fibers will be recruited next, and finally during contractions that require maximal or near maximal force production motor units with type IIX muscle fibers will be recruited. This orderly process of recruiting the motor units with the smallest motor neurons first followed by those with larger motor neurons is called the size principle, which was first described by Henneman in 1965. There is some evidence, albeit mostly from animal studies, that there are exceptions to the rules of the size principle. For example during extremely quick, powerful movements, such as when your cat jumps from the floor to the top of your refrigerator, type IIX fibers may be recruited first. Additionally, it appears possible that during eccentric muscle actions the higher threshold motor units (type IIA or IIX) may be recruited first. What is the importance of this size principle? Well, consider some of the movements from your daily life. When you are picking up a pencil it would not make sense to use the largest, strongest Lab VII - 11 motor units. On the other hand, when you are lifting a large amount of weight adding the force of a very small motor unit would not be very helpful. Recall that type I fibers are especially equipped for aerobic metabolism and type IIX fibers are especially equipped for anaerobic metabolism. Type I fibers are more efficient for several reasons: they use ATP more slowly, they do not need to be stimulated as frequently, and they can use aerobic metabolism (you get more ATP/glucose than anaerobic). So, from an energy standpoint, this orderly recruitment of motor units makes us energetically much more efficient. So, in considering the complexity of a single joint motion, the CNS is doing many things at once. It takes in sensory information about the length of the muscle, the joint angle, the speed of the movement, and the amount of tension being placed upon and created by the muscle. At the same time the CNS alters the total number of motor units fired, as well as altering the firing rate of the motor units so that the appropriate amount of force is being produced. This recruitment of motor units also appears to occur, in humans, in a specific order (Type I IIA IIX). The CNS also recruits the motor units asynchronously in an attempt to avoid fatigue. Furthermore, the recruitment of agonist muscles must be coordinated and the antagonist muscles must be inhibited. All of this is going on for just a simple, single joint movement! Now consider the complexity of a multi-joint movement. The CNS would have to process a large amount of sensory information, and it would need to do so very quickly. Think about pitching a baseball. Several muscle groups are being recruited in a specific sequence. Sensory information must be sent bask to the CNS to interpret where in space and time the body parts are. Then the CNS must be able to predict ahead of time when the arm will be in the right position to release the ball, and then release the ball, with some accuracy, all within several hundredths of a second. Now let us turn our attention to some of the regions of the brain and spinal cord responsible for the control of all of the above occurrences. Neural Control of Movement: The Central Nervous System & Coordination The performance of even the simplest voluntary movements requires complex involvement of the higher brain centers. As we have seen, even a simple arm curl requires that different numbers of motor units must be recruited at different times not to mention that different muscles must also be called upon at different joint angles. The central nervous system must control the timing and amount of recruitment through out all movements. Just anterior to the central sulcus in the cerebrum is the primary motor cortex which is involved in all types of voluntary motor control of the skeletal muscles. The pyramidal (or corticospinal) tracts are tracts of nerve fibers that transmit information, uninterrupted, from the primary motor cortex down the spinal cord to stimulate the motor neurons innervating our muscles. Just anterior to the primary motor cortex is the pre motor cortex, which is involved in the coordination of groups of muscles. The pre motor cortex is associated with many other areas of the brain (basal ganglia, thalamus, primary motor cortex). The neural connections to these areas are outside of the pyramidal tract and are therefore referred to as extrapyramidal tracts. It is believe that the pre motor cortex may be involved in the development of motor skills. The function of the motor cortex in controlling motor outflow would be ineffective without some type of sensory feedback. The sensory somatic Cortex, located just posterior to the central sulcus, is in close connection with the primary motor cortex, providing feedback. The actual comparison of motor output and sensory input is the job of the cerebellum. The cerebellum not only monitors this information but has the ability to predict outcomes and initiate motor output changes. Lab VII - 12 LABORATORY PROCEDURES I. Power, Force, Velocity, and their Relationships to One Another Muscular power can be evaluated during a dynamic action by measuring the transit time of a given resistance over a given distance. This procedure may be used to find the level of resistance needed to provide the optimal power output. Use the arm curl exercise and the timing lights to make this evaluation. Start with a light weight and move the dumbbell through the timing lights as fast as possible. Repeat this procedure with progressively heavier weight. Isolate the elbow flexors and make sure you measure the same range of motion on each attempt. This test would be more appropriate for a lift with a linear movement pattern of the resistance. Use a variety of weights, ranging from very easy to lift up until The heaviest weight the subject can lift one time (1RM). Notes on Calculations: Convert resistance from pounds to Newtons (pounds / 2.2 lb/Kg x 9.8 m/sec2). Convert distance from inches to meters if necessary (0.0254 meters/inch). Power = (Force (N) x Distance (M) ) / Time (Sec) = Watts Distance ___________ Weight Force Time Velocity Power What happened to the velocity of movement as the Force increased? Is this what we would expect? Explain Describe, in your own words what happens to power as the force is increased. Plot your data (power, %1RM) using Microsoft Excel or some other computer graphing software (see appendix) Lab VII - 13 What % of 1RM provides the highest power output? Power | | | | | | | | | | | | | | | | | | | | | Velocity Power | | | | | | | Force What are some practical implications of these relationships to sport performance? How are the Power-velocity and power-force relationships related to the force-velocity relationship we discussed in the previous lab? How might the P-V, P- F, F-V relationships be influenced by fiber types and training? Are there any limitations to the methods we used for assessing power? Lab VII - 14 II. Muscle Endurance and Mechanisms of Fatigue A. Isokinetic Events Isokinetic muscular endurance testing is usually conducted at moderate speeds (of 180 /sec or greater). Following are two isokinetic endurance tests. 1. 50% test - Following an initial test to determine the maximal force production at the desired speed the subject starts a series of maximal contractions until he/she is unable to maintain 50% of the initial maximal force. The test is reported as the number of contractions completed above 50%. This procedure will not be performed. 2. Thorstensson Test - This test of muscular endurance has the subject maximally contract for a given number of repetitions (usually 25 to 100). The mean torque of the first third of contractions is compared to the mean of the last third of contractions to determine a Fatigue Index. The fatigue index reflects the percentage of work output that is maintained. During this procedure we will also evaluate the effects of fatigue on EMG activity - prep your subject for a quadriceps EMG (vastus lateralis). Note: the computer calculates work fatigue (% decline in work output), which is equal to 100% minus the fatigue index. (Mean of last third of contractions) Fatigue Index = x 100 (Mean of first third of contractions) Extensor Data: Power: ________ Work First Third: _________ Work Last Third: _________ Fatigue Index: _________ Flexor Data: Power: ________ Work First Third: _________ Work Last Third: _________ Fatigue Index: _________ If EMG was recorded, what happened to the iEMG activity during the test? If not recorded, what do you think would happen to EMG activity during the course of the test? Would this represent a “central” or “peripheral” mechanism of fatigue? Who would you expect to have a greater fatigue index, a sprinter or a marathoner? In your own words, what does the fatigue index represent? What affect would you expect fiber type to influence fatigue index? Lab VII - 15 B. Isometric Bicep Curl & Mechanisms Of Fatigue. Prepare your subject’s Biceps brachii and attach EMG electrodes. 1. Determine Maximal Voluntary Contraction. Set up the computerized force transducer (similar to a cable tensiometers) so that the subject will be able to perform an isometric biceps curl at a joint angle of approximately 90 to 100. Press start on the computer (which will record both the EMG and force transducer simultaneously). Have the subject pull up on the cable as hard as possible while trying to minimize recruitment of other muscles and muscle groups. Determine the force from the maximal voluntary contraction by clicking and dragging from the baseline to the peak (highest point) on the force recording (Note: make sure that you are recording data from the correct channel), the number in the button bar labeled "delta" will give you the MVC in units of pounds. Then, determine the iEMG integral at the point where maximum force was developed. It is recommended that you have the subject perform this MVC two to three times with about a one minute rest in between attempts (if time is short, one works fine). Delta T: _______ Effort 1: MVC: ___________ pounds Integral: _______ Effort 2: MVC: ___________ pounds Integral: _______ Effort 3: MVC: ___________ pounds Integral: _______ 2. Sustained Submaximal Isometric Contraction. Leave your subject hooked up to the EMG device. You will now have your subject perform an isometric bicep curl while pulling on the force transducer at ~60% of their maximum force until they are unable to maintain this force (alternatively, have the subject hold a dumbbell approximately 60% of the MVC). Start the computer, and have the subject start bringing up the force until it reaches the the desired level (equivalent to 60% of MVC), then start the stopwatch. When the subject can no longer maintain this force, the test is over. After the test review the EMG data and determine the initial EMG activity somewhere in the first 5 seconds after the subject got into position (initial iEMG integral). Make sure to use the same delta T used above. Next, determine the EMG activity at some point during the last 5 seconds of the test (final iEMG integral, before they were unable to maintain their force). It is important to make sure that your final iEMG measurements are taken at the end when the subject was still maintaining the proper force. Next, calculate their normalized iEMG activity as a percent of their maximal voluntary contraction (just like you did in lab 3). 60% of MVC Force: _________ pounds Endurance time: _______________ Initial iEMG integral: ___________ Normalized Initial iEMG: _________ Final iEMG integral: ___________ Normalized Final iEMG: _________ Draw a rough sketch of the EMG tracing during the test. Voltage time Based on all available information, what are possible mechanisms that caused fatigue in this experiment? Can any mechanisms of fatigue can be ruled out? Lab VII - 16 3. Sustained Maximal Isometric Contraction Leave your subject hooked up to the EMG device. Allow your subject to rest for about 5 minutes while you answer questions in your lab manual. You will now have your subject perform a sustained, maximal isometric bicep curl while pulling on the force transducer. Start the computer, have the subject begin to maximally contract and start the stop watch. Have the subject pull as hard as they can for 15-20 seconds while recording both the force and the EMG. After the test review the EMG data and determine the initial EMG activity and force (usually in the first 5 seconds after the start of the contraction). Make sure to use the same delta T used above. Next, determine the EMG activity and force at some point during the last 5 seconds of the test (final iEMG integral, before they were unable to maintain their force). Initial iEMG integral: ___________ Initial Force: __________ Final iEMG integral: ___________ Final Force: __________ Draw a rough sketch of the EMG tracing during the test. Voltage time Based on all available information, what are possible mechanisms that caused fatigue in this experiment? Can any mechanisms of fatigue can be ruled out? Did the mechanisms of fatigue in the two above experiments appear to be similar or different? Explain? B. Endurance during Dynamic Bicep Curls & Mechanisms Of Fatigue. 1. First determine your subject’s 1RM using the following methods: a. determine the subject’s perceived 1RM (ask) b. 5-10 reps at 40-60% of estimated/perceived 1RM c. 1 min rest with light activity d. 3-5 reps at 60-80% of estimated/perceived 1RM e. add a little more weight so that it is slightly below the estimated 1RM and have the subject attempt to lift the weight one time. f. if the lift was successful, have the subject rest 3-5 minutes g. add some more weight and have the subject try and lift it one time. h. repeat steps f & g until the subject fails to lift the weight. The greatest weight the subject successfully lifted one time is the 1RM. Ideally, 1RM is identified within 3-5 attempts. Lab VII - 17 2. Evaluation of muscular endurance by performing reps to failure at a % of the 1RM. Following the testing of an individual’s 1RM strength their endurance can be evaluated by having them go to failure (as many repetitions as possible) with a percentage of their max. We will have our subject perform repetitions until failure at 60% of 1RM. Calculate the appropriate resistance based on your subject’s 1RM. First prep your subject for a Biceps Brachii EMG, and record their EMG activity through out the test procedure. Make sure that your subject maintains proper form for all repetitions. When the subject can no longer lift the weight while using the proper form, the test is over. Assess their peak electrical activity from their first concentric action and their last complete concentric action. Delta T: ____________ Weight at 60% of 1RM __________ Repetitions to failure __________ 1st contration integral ___________ Last contraction integral: ___________ What happened to EMG activity during this endurance test? How can you explain this finding? What model of fatigue best explains these findings? What model(s) of fatigue can be ruled out completely? What other physiological data could have been collected in our fatigue experiments to better identify the possible cause(s) of fatigue? III. Neural Control of Skeletal Muscle and Making Use of Proprioreceptors A. Stretch - Shortening Cycle. Typically a subject can perform a more forceful contraction during a plyometric exercise than during a normal contraction. It is believed that an eccentric loading of the muscle will cause a stretch reflex (involuntary motor unit recruitment) that is added to a voluntary concentric action. If this is true, one would expect greater motor unit recruitment during a plyometric jump than a during a “normal” jump. To evaluate the mechanism by which the stretch-shortening cycle increases muscular force production iEMG data will be measured during a variety of jump activities. Prep the skin on the front of the thigh and place your gelled electrodes over the vastus lateralis muscle. Connect the three electrode wires to the electrode buttons. Once all of the electrical connections (including electrode connections) have been made turn the Power switch on the DAM-50 to on and turn the Input select from ground to AB. Make sure you turn these controls to their off positions before you remove or adjust your electrodes. Record the peak iEMG activity just prior to takeoff and record the jump height for each of the following types of jumps. Use the electronic switch mat to determine when your subject made contact with the ground or left the ground. Delta T: ____________ Lab VII - 18 1. Counter - Movement Jump. Start with feet side by side (no steps), use an active lowering of your body mass and arm swing to produce your jump. Jump Height , integral . 2. No Counter - Movement Jump. Start with feet side by side (no steps), and lower your body mass to a stationary lower position. You can use your arms to produce your jump. Jump Height , integral . 3. Plyometric - Drop Jump. Jump off of both feet immediately after landing from a 33” drop from a box. You can use your arms to produce your jump. Jump Height , integral . Explain a possible mechanism by which use a proprioreceptor might increase motor unit recruitment, and thus enhance jump height immediately following a pre-stretch. Are there any scenarios by which a proprioreceptor might decrease motor unit recruitment, and thus tend to reduce jump height immediately following a pre-stretch? Name elastic components of skeletal muscles, and of the fibers that make them up, that could potentially contribute to the jump Explain any differences in jump height and EMG activity between jump 1 and jump 2. Explain any differences in jump height and EMG activity between jump 2 and jump 3. B. Proprioceptive Neuromuscular Facilitation. To evaluate the effect of PNF procedures on flexibility (range of motion, ROM) we will use a fleximter to measure ROM before and after two PNF procedures. Have your subject lie on their back with their legs straight but relaxed on the exam table. Place the fleximeter on the front of the tibia of your subject and press the zero button. This will set the fleximter to register this joint position as a 0° angle reference position of the leg to be tested. Use the knobs on the outside of the fleximter to zero the device with the leg in the relaxed position on the table. While keeping their knee straight and their ankle flexed at 90°, have your subject flex their hip as far as possible (keeping their hips on the table). Assist your subject in holding this position for 5 seconds. In this position record the joint angle on the fleximter (still held on the front of the tibia). Maximal Hip Flexion Joint angle __________ Lab VII - 19 B. Following a short rest period perform a Hold-Relax PNF Procedure. To do this, have your subject assume the hip flexion position previously attained. A partner will then place support on the elevated leg with one hand on the subject’s heel and the other on the subject’s knee. First, the subject’s leg will be held by the partner in position for 10 seconds of passive stretch. On the command of the partner the subject will perform a 10 second isometric action of the hip extensors (hamstrings). The subject will gradually build up tension for the first 3-4 seconds and sustain maximal tension for the final 4-6 seconds. Following the contraction, the partner will provide a 30 second passive static stretch. Following this procedure measure the hip flexion angle using your fleximter. Hip flexion joint angle following Hold-Relax PNF __________ Was your subject able to stretch further? If so,why? What proprioreceptor(s) are involved in this PNF procedure? What type of reflex is involved in this PNF procedure? Draw and label the reflex arc for this reflex. C. In the final phase of this experiment you will perform a Hold-Relax with Agonist Contraction PNF procedure. This procedure is almost the same as the Hold-Relax procedure, except that following the strong hamstring contraction the partner will instruct the subject to activate the hip flexors. The subject will try to pull their leg up to their head. Again the contraction is sustained for 10 seconds (first 4 building up tension and the last 6 hard). Following this procedure measure the hip flexion angle using your fleximter. Stretch the leg while the subject is still contracting. Before starting get a new baseline joint angle. New baseline joint angle (before performing this procedure): __________ Hip flexion joint angle following hold-relax with agonist contraction PNF __________ Was your subject able to stretch further? If so why? What proprioreceptors are involved in the 1st part of this PNF procedure? The 2nd part? What type of reflexes are involved in the 1st and 2nd parts of this PNF procedure? Lab VII - 20 Draw and label the reflex arcs involved in the 1st and 2nd parts of this PNF procedure. Because these procedures use the inverse stretch reflex and reciprocal inhibition, what would you expect to happen to hamstring EMG activity during each of these PNF procedures? Why? IV. Neuromuscular Coordination. In this experiment you will examine the recruitment pattern of two muscle groups (abdominals and hip flexors) during different variations of sit-ups. In order to avoid recording the electrical activity of the heart, prep the skin over the right side of the rectus abdominus and the right upper rectus femoris muscles for electrode application. Place your gelled electrodes over the muscles and connect the three electrode wires to the electrode buttons. Once all of the electrical connections (including electrode connections) have been made turn the Power switch on the DAM-50 to on and turn the Input select from ground to A-B. Make sure you turn these controls to their off positions before you remove or adjust your electrodes. Record IEMG activity through the normal range of motion and record the activation patterns below. Try to time the movements (2-3 seconds up and 2-3 seconds down) so that EMG activity can be related to body position (angle). Draw the iEMG activity of the hip flexors and abdominals duringeach and record the concentric and eccentric iEMG activity over the peak one second (delta T = 1 sec) of activity. A. Straight Leg sit - up. With legs straight out on the floor and hands across the chest and perform a sit - up from the down to up and up to down position. Hip flexors Concentric integral: ___________ Eccentric integral: ___________ Down Up Down Abdominals Concentric integral: ___________ Eccentric integral: ___________ Down Up Lab VII - 21 Down B. Bent - Knee sit - up. Bend your knees with your feet flat on the floor and your hands across your chest and perform a sit - up from the down to up and up to down position. Hip flexors Concentric integral: ___________ Eccentric integral: ___________ Down Up Down Abdominals Concentric integral: ___________ Eccentric integral: ___________ Down Up Down C. Crunch. Lie on your back with legs supported on a bench with thighs at a 90° angle to the floor. Place your hands across your chest and pull your chest up to your thighs, from the down to up and up to down position. Hip flexors Concentric integral: ___________ Eccentric integral: ___________ Down Up Down Abdominals Concentric integral: ___________ Eccentric integral: ___________ Down Up Down Which of these activities best isolated the abdominal muscles? Which of these activities best activated the abdominal muscles? Considering what you know about training and sport specificity, what do you think the role of “isolating” a muscle group is relative to athletic performance or performance of daily living activities? How did the CNS know to change the recruitment pattern of these two muscles when performing the different types of actions? Name the major parts of the brain responsible for changing the recruitment pattern of these two muscles when performing the different types of actions? Lab VII - 22 Study questions 1. Define muscular strength, muscular power, and muscular endurance? Name a sport or type of athlete that would require large amounts of each of these. 2. What properties of skeletal muscle would be major determinants of muscular strength? Muscular power? Muscular endurance? 3. Explain in your own words, while being as specific as possible, the procedures for determining a subject’s 1 RM. 4. Explain how wave summation, motor unit summation, asynchronous motor unit recuruitment, and the size principle relate to muscular force production and to the experiments performed in this lab. 5. Is muscular power or strength probably more important for most athletes? Explain. 6. Draw graphs depicting the following relationships: a. The length-tension curve b. The force-velocity curve (include both concentric and eccentric) c. The power-velocity curve (include both concentric and eccentric) d. The power-load (resistance) curve for concentric actions 7. What mechanism(s) of fatigue were most likely involved in our experiments? How could you tell based on EMG activity? 8. To what extent can EMG help your determine the possible causes of fatigue? 9. Does lactic acid cause fatigue? Explain your answer. Lab VII - 23 10. Explain, in your own words, and in some detail, six possible causes of fatigue. 11. Explain, in your own words, how fatigue is related to endurance performance and protection of the muscle fibers. 12. Explain three mechanisms by which Pi (inorganic phosphate) can cause fatigue. 13. Explain three mechanisms by which calcium release, reuptake, and/or sensitivity may influence fatigue. 14. What is muscle wisdom? During what types of contractions does it occur? How does muscle wisdom potentially relate to fatigue. 15. Draw a diagram that shows all of the parts of a generic reflex arc and how they inter-relate. 16. What are some practical uses of proprioreceptors in athletic and clinical settings? 17. Briefly, explain the procedures for the hold-relax PNF procedure. What is the theory behind how this procedure improves flexibility? 18. Briefly, explain the procedures for the hold-relax with agonist contraction PNF procedure. What is the theory behind how this procedure improves flexibility? Lab VII - 24 19. How do the following relate to muscular force production: wave summation, multiple motor unit summation, asynchronous motor unit recruitment, size principle, and golgi tendon organs? 20. What are the roles of the following in the neural control of movement: primary motor cortex, premotor cortex, sensory motor cortex, cerebellum, corticospinal tracts, and extrapyramidal tracts? 21. How does the stretch-shortening cycle improve jump height, in theory? 22. During our “jumping” experiment we had our subject jump with or without a counter-movement, and we also had our subject perform a “drop jump”. We recorded EMG activity from the vastus lateralis and jump height during each. Here are some sample results from these experiments. Briefly, explain (a) possible mechanisms why the subject jumped higher with counter-movement than without, and (b) why the subject jumped higher during the drop jump than the countermovement jump. You MUST explain the iEMG information as part of your answers. (Note: this is an old test question) Drop-jump With counter-movement No counter-movement jump iEMG activity 7.0 x 10-4 5.0 x 10-4 5.0 x 10-4 jump height 25 inches 22 inches 17 inches 23. When performing a drop jump from a great height we sometimes observe a decrease in EMG activity and a decrease in jump height compared to normal jumps. What is a possible cause of such a finding? (hint: think reflexes) 24. Describe how to use our data acquisition hardware to determine how much electrical activity we are observing on an EMG. Include how we start, stop, scale, etc. Also, what pieces of information do we record, and what do these numbers represent? Lab VII - 25