Oxidation-Reduction Reaction
advertisement
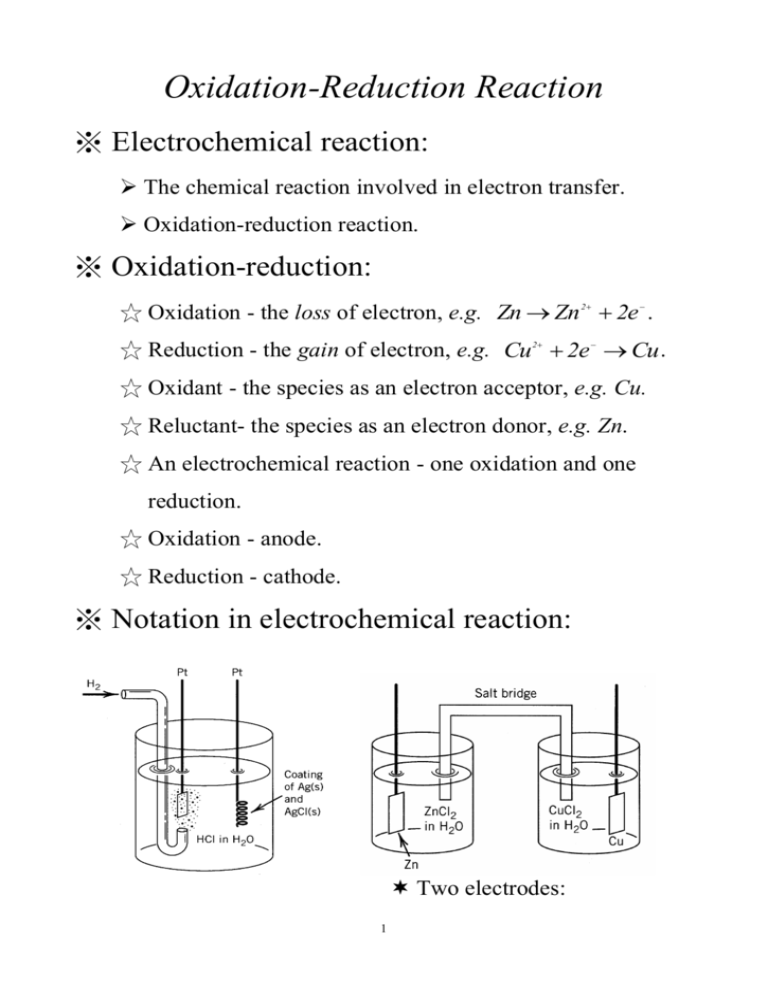
Oxidation-Reduction Reaction ※ Electrochemical reaction: The chemical reaction involved in electron transfer. Oxidation-reduction reaction. ※ Oxidation-reduction: ☆ Oxidation - the loss of electron, e.g. Zn Zn 2e . 2 ☆ Reduction - the gain of electron, e.g. Cu 2e Cu . 2 ☆ Oxidant - the species as an electron acceptor, e.g. Cu. ☆ Reluctant- the species as an electron donor, e.g. Zn. ☆ An electrochemical reaction - one oxidation and one reduction. ☆ Oxidation - anode. ☆ Reduction - cathode. ※ Notation in electrochemical reaction: Two electrodes: 1 anode/cathode. Electrolyte solution. Salt bridge (not necessary). ☆Notation: Oxidation Reduction or Oxidation Reduction with liquid junction (salt bridge): A A e.g. CuCu 0.01m Ag 0.02m B xm ym B Ag without liquid junction (salt bridge): A C common ion in A and B); g. Pt H 2( g ) HCl xm B (C contains (1 m ) AgCl Ag ( s) ※ Types of electrochemical cell: ★ Voltaic (Galvanic) cell: battery that store electrical energy. spontaneous reaction. electrons flow from the anode to the cathode via an external conductor. ★ Electrolytic cell: requiring an external source of electrical energy for operation. non-spontaneous reaction. 2 Anode Cathode Voltaic - + Electrolytic + - ※ Cell potential: standard condition @P=1bar , T=25℃and C=1m. Ecell measured in reduction potential. E E cell anode Ecell > 0 E cathode . voltaic cell. ※ Standard electrodes: Hydrogen electrode: Pt H ( 1bar ) H ( 1m ) ( aq ) 2 Calomel electrode: Hg Hg Cl 2 3 2( S ) E0=0 V. Cl ( 0 .1m ) E0=0.3338 V. ( aq ) ※ Thermodynamics of electrochemical cells: ☆ Gibbs energy: G w 0 non PV G zFE for any standard reaction. 0 0 where F=96500 C/mole and z =charge number for cell reaction. E0 > 0, spontaneous reaction. E 0 RT n K 0 zF ☆ Electromotive force in non-standard condition: For aA bB yY zZ reaction: EE 0 Y Z n zF A B y z a b RT @25℃, E E 0 0.0592 z called the Nernst equation. Y Z og A B y z a b ☆ Entropy: G S T P E S zF T G S T P P ☆ Enthalpy: H G T S 4 E H zF E T T ※ Nernst potentials: ☆ Situation I: K+ and Cl- are both permeable At equilibrium: [Cl-] 1=[Cl-] 2; [K+] 1=[K+] 2 0 ☆ Situation II: K+ is only permeable Some K+ ions across membrane from left to right. A potential established to prevent more K+ ions from crossing. The potential is called the Nernst potential. RT F n C1 C2 Concentration change on both sides is very little. ※ Types of electrochemical cells: (I) Chemical cells: the cell is involved in a net chemical change. 5 Cells in which the chemical reaction involves the electrodes: 2 EE 2 0 e.g. Zn Zn Cu Cu RT F Zn n Cu 2 2 Redox cells: both the oxidized and reduced species in solution and their interconversion is effected by an inert electrode. e.g. Pt H H 2 2 EE 3 Fe , Fe Pt ( 1m ) 0 Fe n Fe 2 RT 3 F (II) Concentration cells: the cell is involved in a change of concentration. e.g. Pt H HCl (m1 ) 2 HCl E H Pt ( m2 ) 2 RT n F m2 m1 ※ Applications of EMF measurements: (A) pH determination: Pt H ( 1bar ) H ( a ) Hg Cl H 2 oxidation: 1 reduction: 1 overall: pH 2 2 H e 2( g ) 2( S ) Hg Cl ( 1m ) (S) Hg Cl e Hg Cl 2 1 H 2 H 2 2( g ) 1 0 Hg Cl Hg Cl H 2 2 2(S) E 0. 2 8 0 2 0 .0 5 9 1 E 0 . 2802V (S) 2 @25℃. 6 (S) (B) Determination of activity coefficient: Pt H ( 1bar ) HCl 2 1 oxidation: H 2 AgCl Ag Cl ( aq ) (S) (S) H e 2( g ) reduction: AgCl e Ag Cl overall: 1 EE RT 0 H 2 H AgCl Ag Cl H 0 H+ m;a Cl (S) (S) n a a E F a 2( g ) (S) (S) 2RT Cl n m F 2RT n F m (C) Determinatio n of dissociation constant, HA: Pt H ( 1bar ) HA ( m ), NaA ( m ) AgCl Ag NaCl ( m ) 2 oxidation: 1 1 2 H 2 H e 2( g ) 7 (S) (S) 3 reduction: AgCl e Ag Cl overall: 1 EE RT 0 2 H E RT F E 0 RT H+ (E E ) F RT E 0 Cl A - n K a n[ mA RT RT - n[ n[ a a a a ] a a n K Cl - A- ] n[ HA Cl - A - RT 2 ( aq ) AgCl Ag (S) 8 (S) A- HA A- n[ F ] n K - (D) Determination of solubility product: Cl ( 1bar ) HCl Cl - a m m ] m HA H+ HA F F mHA mCl RT F Cl - HA (S) (S) a a n[ ] a F 0 AgCl Ag Cl H n a a F 0 2( g ) (S) (S) ] Cl - HA A- a oxidation: Cl 1 2 Cl 2( g ) e reduction: AgCl e Ag Cl (S) (S) overall: AgCl Ag Cl + (S) EE 0 RT F n 1 a Ag a Cl E 0 RT F 9 n a a Ag E 0 Cl RT F n K SP








