Structure-Guided Directed Evolution of Highly Selective P450
advertisement

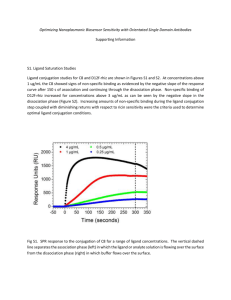
Supplementary Information Structure-Guided Directed Evolution of Highly Selective P450-based Magnetic Resonance Imaging Sensors for Dopamine and Serotonin Eric M. Brustad1, Victor S. Lelyveld2, Christopher D. Snow1, Nathan Crook1, Sang Taek Jung1, Francisco M. Martinez5, Timothy J. Scholl5, Alan Jasanoff2-4* and Frances H. Arnold1* 1Division of Chemistry and Chemical Engineering, California Institute of Technology, Pasadena, CA, USA 2Department of Biological Engineering, Massachusetts Institute of Technology, Cambridge, MA, USA 3Department of Brain and Cognitive Sciences, Massachusetts Institute of Technology, Cambridge, MA, USA 4Department of Nuclear Science and Engineering, Massachusetts Institute of Technology, Cambridge, MA, USA 5Department of Physics and Astronomy, University of Western Ontario, London, ON, Canada Supplementary Fig. 1. High-throughput optical screening and directed evolution for Improved neurotransmitter binding in BM3h. A customized optical screening method was developed to evolve ligand binding within BM3h1. Fatty acids are so-called ‘type I’ substrates, inducing a hypsochromic shift in the P450’s characteristic Soret absorbance band associated with catalytically important changes in the heme electronic environment2; 3. The bathochromic shift observed upon DA binding (from λmax = 419 nm to 425 nm) is consistent with ‘type II’ ligand binding, indicative of DA coordination to the heme iron 2. Binding isotherms can be generated by monitoring absorbance changes as a function of ligand concentration. For panels a - c, we show data from BM3h-2G9C6 as a representative example of P450 ligand binding measurements described in the Methods. a) Characteristic P450-BM3h Soret band with MAX at ~ 419 nM (solid line). In the presence of saturating biogenic amine neurotransmitters such as serotonin (5HT), this absorbance is red shifted to ~425 nm (dashed line). b) Difference absorbance spectra are acquired by subtracting ligand bound and unbound Soret spectra. Maximum absorbance changes at 415 nm and 435 nm (triangles) were used to generate binding isotherms (panel c) at each ligand concentration. c) Typical binding isotherm. d) Directed evolution protocol: DNA libraries were constructed by active site-saturation mutagenesis using degenerate NNK oligonucleotides. Libraries were transformed into competent BL21-(DE3) E. coli. Individual BM3h variants colonies were picked, expressed in 96 well blocks, and harvested cell lysates were distributed into 3 microtitre 96 well plates. As shown in the middle of this panel, BM3h expression was readily observed by the characteristic red color (red circles) consistent with heme bound proteins. Each library was co-screened against DA, 5HT and NE to allow positive/negative for improved affinity and selectivity. Promising variants were analyzed for ligand dependent MRI changes. This process was repeated for multiple cycles to yield final evolved BM3h variants. a Compound R1 R2 R3 KD (M) dopamine (DA) OH OH NH2 3.3 0.6 tyramine OH H NH2 7.0 1.2 m - hydroxyphenethylamine H OH NH2 18.0 1.7 phenethylamine H H NH2 17.9 0.8 4-ethylcatechol OH OH H no binding b Compound R1 R2 KD (M) serotonin (5HT) OH H 0.7 0.1 tryptamine H H 0.6 0.1 N - acetylserotonin OH Ac No binding O - methylserotonin OCH3 H 0.4 0.1 melatonin OCH3 Ac No binding R3 L-tryptophan H No binding 5-hydroxytryptophan OH - No binding 5-hydroxyindoleacetic acid - - No binding 5-hydroxyindoleacetic acid Supplementary Fig. 2. Structure-activity relationship of BM3h neurotransmitter binding. Type II ligand binding in P450s is indicative of direct ligand coordination to the heme iron. To assess the nature of this binding, a variety of commercially available dopamine and serotonin homologues, modified at the exocyclic hydroxyl groups and aliphatic amine moieties, were screened for binding to variants BM3h-B7 (a) and BM3h-2G9C6 (b). Modification of the exocyclic hydroxyls has a minimal effect on ligand binding whereas deletion or acetylation of the neurotransmitter amine functionality abrogates ligand binding. 5HT precursors such as tryptophan and hydroxytryptophan show no binding to BM3h-2G9C6 as measured by absorbance. In addition, serotonin metabolites such as 5-hydroindoleacetic acid and melatonin do not bind BM3h-2G9C6. a. b. c. d. e. f. Supplementary Fig. 3. Electron density of BM3h-bound DA and 5HT. Maximum likelihood weighted electron density maps of protein-bound neurotransmitter and the heme cofactor. (a) BM3h-2G9C6 + serotonin (b) BM3h-2G9 + serotonin (c) BM3h-9D7 + dopamine (d) BM3h-8C8 + dopamine (e) BM3h-8C8 + serotonin and (f) BM3h-B7 + dopamine. All panels were contoured at = 1.5. Supplementary Fig. 4. Global protein structure in evolved BM3h variants. Neurotransmitter binding in P450-BM3 does not contribute to the closed conformation. Panels a – c and g – i show alignments of BM3h variants to the open wild type BM3h conformation (green) taken from PDB:2IJ2 4. Panels d – f and j – l show residue-by-residue C RMSD values for the corresponding alignment above. The closed conformation in the presence of cognate fatty acid ligand is shown in (a), taken from PDB:1JPZ5) and is characterized by three regions that show significant substrate-induced conformational changes: the N-terminal beta subdomain (region 1), the tip of the active site capping F- and G-helices (region 2) and the hinge region at the bottom of the G-helix (region 3). The corresponding regions are also marked in the residue-by-residue RMSD graph (d). Panels (b/e) and (d/f) show minor deviations from open wild type for BM3h-8C8 in the absence (b/e) or presence (c/f) of dopamine. Small movements in region 1 are observed in both structures, regardless of ligand binding. Serotonin bound BM3h-2G9 (g/j) closely resembles the open wild type P450 structure with little movement observed in all three regions. BM3h-2G9C6 in the absence (h/k) or presence (i/l) of serotonin shows movement in region 1 and 2, but no change in the hinge region (3). Observed backbone movements are likely crystal packing artifacts. For example, in the BM3h-2G9C6 structure in the absence of ligand (h), alternate conformations can be observed between both chains in the asymmetric unit (colored brown and gold in panel h) suggesting that these movements are likely influenced by interactions within the protein crystal. The absence of ligand dependent conformational changes suggests that global protein backbone changes are not required for or induced by neurotransmitter binding in evolved fMRI sensors. a. b. Supplementary Fig. 5. Results from screening double NNK libraries in parent scaffold BM3h-B7. a) Apparent KD values for 5HT-selective hits observed during screening. KD values (given in M) are colorcode from greatest affinity (green) to least affinity (red). Apparent K D titrations for these experiments were performed in clarified cell lysates. Typically these values are at least 3-fold lower than KD titrations performed on purified BM3h variants. b) Soret optical shift observed for BM3h variant 3DB10 in the absence of ligand (dashed line) and presence of ligand (dotted line). For comparison, the Soret band for wild type BM3h is also shown (solid line) with a MAX at ~ 419 nm. BM3h-3DB10 is slightly red-shifted even in the absence of ligand. Electronic or structural changes at the heme center that cause this shift may also lead to the decrease in r1 observed for this mutant. Supplementary Fig. 6. Heterogeneous active site density in unbound BM3h structures. a) For reference, a histogram of relaxivity (r1) for several ligand-free variants described in this work is shown. b) Normalized Soret absorbance spectra for all BM3h variants in the absence of ligand (solid line) is consistent with a low spin heme center (max = 419 nm). For comparison, high spin wild type BM3h, bound to saturating lauric acid (a model fatty acid substrate) to induce spin shift is shown (dotted line) with a max ~ 390 nm. Based on these data, changes in the ferric iron spin state are probably not involved in relaxivity differences. Supplementary Fig. 6. Heterogeneous active site density in unbound BM3h structures: continued. Averaged kicked omit maps were generated to display axial heme density in ligand-free BM3h crystal structures (heme = 1.5 and axial = 1.0). Averaged kick omit maps were generated using the calculate maps tool of the PHENIX crystallographic refinement software package 6. Panels c – g show heme bound omit-density for BM3h variants in order of increasing r1: BM3h-8C8 (c), wild type BM3h (d; taken from pdb:2IJ24), BM3h-2G9 (e), BM3h-9D7 (f), and BM3h-2G9C6 (g). Top and bottom panels for each structure refer to chains A and B of asymmetric unit, respectively. Amorphous regions of proximal-heme density among existing BM3h structures have been attributed to mixed occupancy water molecules. We are unable to rule out the possibility that increased density in high-r1 variants is the result of promiscuous binding to components of the crystallization mother liquor. However, the most likely contaminant, acetate (a crystallization co-salt), fails to account for the total observed electron density nor does acetate yield changes in the P450 Soret spectrum upon titration. Nevertheless, the observed trend of increasing electron density around the heme iron may reflect the improved relaxivity observed in variants such as BM3h-2G9C6. Supplementary Fig. 7. B factor analysis of BM3h variants in the absence of ligand. a - c) Active sites for BM3h variants BM3h-8C8 (a), wild type (b, taken from pdb:2IJ2)4 and BM3h-2G9C6 (c). In the high relaxivity mutant, BM3h-2G9C6, several amino acid side chains within the active site, including the mutation at L437Q, are observed to exist in multiple conformations within the crystal structure. (chain A is shown in green, chain B is shown in orange). d – f) The same active site structures from above are shown color-coded for B factor. A color bar is shown under panel e. For the higher relaxivity mutant, BM3h-2G9C6, increased mobility is observed for the regions shown. g) Plots of α-carbon B factor versus relaxivity (r1) are shown for select active site mutations as well as the proximal heme ligand, cys400, for reference. B factors were taken from ligand free crystal structures solved during this work (BM3h-8C8, BM3h-2G9, BM3h-9D7 and BM3h-2G9C6) as well as ligand free wild type protein (pdb: 2IJ2)4. To compare B factors between structures, we normalized C B-factor with the average heme B-factor to account for differences in resolution. Others have applied similar normalization strategies to compare different protein structures7 and normalizations to other BM3h residues provided similar results. Plotting residue-by-residue B-factors against ligand-free r1 showed three regions with marked correlation between these two properties (panels g and h). h) The residue-by-residue squared correlations (R2) of α-carbon B factor and relaxivity, such as those shown in panel g, are shown for the entire protein sequence with a sliding average over 5 residues (solid line). In addition, the slope of the linear regression of B factor versus relaxivity used to generate R2 is also shown (dotted line). Three regions, highlighted, appear to demonstrate an observed correlation (R2) between increasing local B factor and the improvements in relaxivity observed in evolved BM3h variants. These regions include the active site I-helix including residues 263 – 273 (green), the active site loop comprised of residues 319 – 329 (blue), as well as the active site loop comprised of residues 425 – 440 (red), which includes the mutation L437Q (highlighted in panel c) in the highest relaxivity variant, BM3h-2G9C6. For comparison, one region that demonstrates large deviations in B factor with poor correlation relative to relaxivity changes includes the loop linking the F and G helices (residues ~ 180 – 200), which is known to be highly mobile and often poorly structured in various BM3h structures. As shown in panels i and j, these regions (color coded to match panel h) are located within the active site of the protein, near the heme center, and flank 2 of the 3 largest solvent channels (shown by arrows) leading to the heme. Supplementary Fig. 8. NMRD analysis of 5HT- and DA-binding BM3h mutants. Purified BM3h protein T1 molar relaxivity measured in a field cycling relaxometer at 25°C as a function of applied magnetic field strength (solid circles), shown here as proton Larmor frequency, and best-fit Solomon and Bloembergen dipolar coupling models of a single bound water (q = 1) in the first coordination sphere, with the following fitted parameters (inset): effective proton-ion distance r (units in Å), first coordination sphere bound water residence time τM (units in µs), and frequency-independent electronic relaxation time τS (units in ns). The fitting procedure assumed a uniform population of low spin ferric ions (total electron spin, S = 1/2), a Stokes-Einstein radius of 3.5 nm (corresponding to a rotational correlation time, τR = 39 ns), a modeled diamagnetic contribution based on apotransferrin8, and that the scalar contribution to the overall relaxation process was negligible. Supplementary Table 1. Data collection and refinement statistics for BM3h-8C8 crystals pdb accession # BM3h-8C8 (no ligand) 4DTY BM3h-8C8:DA complex 4DTZ BM3h-8C8:5HT complex 4DTW Data collection Space group Wavelength P 1 21 1 1.033 P 1 21 1 1.033 P 1 21 1 1.033 58.75, 145.66, 63.53 97.10 58.70, 146.37, 63.98 97.59 58.61, 146.25, 64.06 97.51 38.5 - 1.45 (1.45 - 1.53) * 5.6(55.5) 10.3(2.1) 93.8(93.0) 3.4(3.3) 38.9 - 1.55 (1.55 - 1.63) * 5.1(44.8) 11.8(2.3) 95.7(98.1) 2.6(2.6) 38.9 - 1.8 (1.8 – 1.9) * a, b, c (Å) a b g () Resolution (Å) Rmerge I / σI Completeness (%) Redundancy 7.8(58.0) 9.0(1.9) 92.5(95.0) 2.4(2.4) Refinement Resolution (Å) 36.4 - 1.45 37.4 - 1.55 38.9 - 1.8 No. reflections 165582 140219 86403 Rwork / Rfree 0.18 / 0.22 0.18/0.22 0.19/0.25 No. atoms Protein 7370 7343 7308 Ligand/ion 86 108 112 Water 1036 1098 1059 B-factors Protein 16.9 15.9 17.7 Ligand/ion 9.87 9.6 12.3 Water 27.8 28.0 29.6 R.m.s. deviations Bond lengths (Å) 0.027 0.027 0.023 2.21 2.22 1.97 Bond angles () *All data sets were collected from single crystals. Highest-resolution shell is shown in parentheses. Supplementary Table 2. Data collection and refinement statistics for BM3h-B7 crystals pdb accession # BM3h-B7:DA complex 4DU2 Data collection Space group wavelength P 1 21 1 1.033 a, b, c (Å) a b g () Resolution (Å) Rmerge I / σI Completeness (%) Redundancy 58.73, 147.23, 63.22 98.11 39.0 - 1.9 (1.9 – 2.0) * 5.1(44.0) 11.8(1.9) 94.1(93.0) 2.6(2.4) Refinement Resolution (Å) 39.0 - 1.9 No. reflections 74344 Rwork / Rfree 0.20/0.25 No. atoms Protein 7040 Ligand/ion 108 Water 436 B-factors Protein 24.2 Ligand/ion 17.5 Water 31.1 R.m.s. deviations Bond lengths (Å) 0.023 1.89 Bond angles () *All data sets were collected from single crystals. Highest-resolution shell is shown in parentheses. Supplementary Table 3. Data collection and refinement statistics for BM3h-9D7 crystals pdb accession # BM3h-9D7 (no ligand) 4DUA BM3h-9D7:DA complex 4DUB Data collection Space group wavelength P 1 21 1 0.979 P 1 21 1 1.000 58.83, 152.30, 61.78 94.58 58.91, 153.58, 61.10 95.10 39.2 - 2.0 (2.0 - 2.1) * 8.6(60.7) 10.1(2.2) 97.5(97.7) 3.4(3.5) 39.1 - 1.7 (1.7 - 1.8) * 4.8(66.3) 13.7(2.0) 97.7(97.9) 3.5(3.5) a, b, c (Å) a b g () Resolution (Å) Rmerge I / σI Completeness (%) Redundancy Refinement Resolution (Å) 38.4 - 2.0 38.6 - 1.7 No. reflections 67311 109611 Rwork / Rfree 0.16 / 0.21 0.16/0.20 No. atoms Protein 7280 7328 Ligand/ion 95 108 Water 516 731 B-factors Protein 24.9 22.5 Ligand/ion 22.2 14.8 Water 32.0 30.5 R.m.s. deviations Bond lengths (Å) 0.023 0.028 1.90 2.09 Bond angles () *All data sets were collected from single crystals. Highest-resolution shell is shown in parentheses. Supplementary Table 4. Data collection and refinement statistics for BM3h-2G9C6 crystals pdb accession # BM3h-2G9C6 (no ligand) 4DUD BM3h-2G9C6:5HT complex 4DUE Data collection Space group wavelength P 1 21 1 1.000 P 1 21 1 1.033 58.73, 153.30, 60.98 94.68 29.8 – 1.85 (1.85 – 1.95) * 7.4(59.3) 9.7(2.0) 98.6(99.1) 3.5(3.5) 58.61, 148.42, 64.29 98.88 38.4 - 1.7 (1.7 – 1.79) * a, b, c (Å) b ) Resolution (Å) Rmerge I / σI Completeness (%) Redundancy 4.9(65.1) 15.1(1.8) 97.3(98.3) 3.4(3.5) Refinement Resolution (Å) 29.8 – 1.85 38.4 - 1.7 No. reflections 84485 108321 Rwork / Rfree 0.17 / 0.21 0.17 / 0.21 No. atoms Protein 7497 7287 Ligand/ion 86 112 Water 562 836 B-factors Protein 21.3 21.1 Ligand/ion 12.3 16.3 Water 28.2 31.6 R.m.s. deviations Bond lengths (Å) 0.025 0.027 1.95 2.16 Bond angles () *All data sets were collected from single crystals. Highest-resolution shell is shown in parentheses. Supplementary Table 5. Data collection and refinement statistics for BM3h-2G9 crystals pdb accession # BM3h-2G9 (no ligand) 4DUC BM3h-2G9:5HT serotonin complex 4DUF Data collection Space group wavelength P 1 21 1 1.000 P 1 21 1 0.979 a, b, c (Å) b ) Resolution (Å) Rmerge I / σI Completeness (%) Redundancy 58.65, 153.75, 60.98 94.82 29.2 – 1.9 (1.9 – 2.0) * 8.9(42.5) 6.3(2.0) 95.5(78.9) 3.4(3.0) 79.69, 150.49, 87.71 90.32 29.6 - 1.8 (1.8 – 1. 9) * 6.3(51.3) 11.5(2.9) 98.1(96.0) 3.5(3.3) Refinement Resolution (Å) 29.2 – 1.9 29.4 - 1.8 No. reflections 73543 177347 Rwork / Rfree 0.18 / 0.23 0.16 / 0.20 No. atoms Protein 7270 14906 Ligand/ion 86 224 Water 568 1101 B-factors Protein 19.7 16.0 Ligand/ion 12.7 15.1 Water 20.1 24.1 R.m.s. deviations Bond lengths (Å) 0.022 0.026 1.91 2.07 Bond angles () *All data sets were collected from single crystals. Highest-resolution shell is shown in parentheses. Supplementary Table 6. Ramachandran statistics. Structure Crystallization Crystallization condition a,b conditions, MOLREP template molecular Rfree Template c 8C8 (no ligand) 8C8 + dopamine 8C8 + serotonin B7 + dopamine 9D7 (no ligand) 9D7 + dopamine 2G9 (no ligand) 2G9 + serotonin 2G9C6 (no ligand) 2G9C6 + serotonin 0.1 M Tris, pH 8.5 0.2 M MgCl2, 22 % PEG 3350 0.1 M Tris, pH 8.2 0.2 M MgCl2, 20 % PEG 3350 0.1 M Tris, pH 8.5 0.2 M MgCl2, 18 % PEG 3350 0.1 M Tris, pH 8.2 0.2 M MgCl2, 19 % PEG 3350 0.1 M Na cacodylate, pH 5.5, 0.14 M Ca(Ac)2, 14 % PEG 8000 0.1 M Na cacodylate, pH 5.5, 0.14 M Ca(Ac)2, 14 % PEG 8000 0.1 M Na cacodylate, pH 5.5, 0.14 M Ca(Ac)2, 14 % PEG 8000 0.1 M Na cacodylate, pH 5.5, 0.14 M Ca(Ac)2, 14 % PEG 8000 0.1 M Na cacodylate, pH 5.5, 0.14 M Ca(Ac)2, 14 % PEG 8000 0.1 M Na cacodylate, pH 5.5, 0.14 MgCl2, 13 % PEG 3350 replacement models and % Ramachandran outliers (total) 0.1 % ( 1 ) % Ramachandran favored 97.8 % 0.0 % ( 0 ) 97.9 % 8C8 + dopamine 2IJ2 (WT) 8C8 + dopamine New Rfree 8C8 + dopamine 8C8 + dopamine 9D7 + dopamine 8C8 + dopamine 8C8 + dopamine 9D7 + dopamine 0.2 % ( 2 ) 97.9 % 0.2 % ( 2 ) 96.6 % 0.1 % ( 1 ) 97.5 % 2IJ2 (WT) New Rfree 0.0 % ( 0 ) 97.7 % 2IJ2 (WT) New Rfree 0.1 % ( 1 ) 97.8 % 2IJ2 (WT) New Rfree 0.0 % ( 0 ) 97.3 % 2IJ2 (WT) New Rfree 0.0 % ( 0 ) 97.8 % 2G9C6 (no ligand) 2G9C6 (no ligand) 0.0 % ( 0 ) 97.3 % crystallization conditions involved the addition of 1 L protein at 15 mg/mL + 1 L mother liquor listed in the table. aAll bCrystal drops were microseeded with protein crystals grown in the absence of seeding. Seeding crystals usually grew in conditions containing slightly higher PEG concentrations (+ 4 to 6 %) with same salt and buffer concentrations. cIn structures where molecular replacements models were generated from other protein models from this work (i.e. not from wild type, WT), RFree data sets were carried over to minimize over fitting of the data. Supplementary References: 1. Shapiro, M. G., Westmeyer, G. G., Romero, P. A., Szablowski, J. O., Kuster, B., Shah, A., Otey, C. R., Langer, R., Arnold, F. H. & Jasanoff, A. (2010). Directed evolution of a magnetic resonance imaging contrast agent for noninvasive imaging of dopamine. Nat. Biotech. 28, 264-270. 2. Schenkman, J. B., Remmer, H. & Estabrook, R. W. (1967). Spectral Studies of Drug Interaction with Hepatic Microsomal Cytochrome. Mol. Pharma. 3, 113-123. 3. Macdonald, I. D. G., Munro, A. W. & Smith, W. E. (1998). Fatty Acid-Induced Alteration of the Porphyrin Macrocycle of Cytochrome P450 BM3. Biophys. J.74, 3241-3249. 4. Girvan, H. M., Seward, H. E., Toogood, H. S., Cheesman, M. R., Leys, D. & Munro, A. W. (2007). Structural and Spectroscopic Characterization of P450 BM3 Mutants with Unprecedented P450 Heme Iron Ligand Sets. J. Biol. Chem. 282, 564-572. 5. Haines, D. C., Tomchick, D. R., Machius, M. & Peterson, J. A. (2001). Pivotal role of water in the mechanism of P450BM-3. Biochemistry 40, 13456-65. 6. Adams, P. D., et al. (2010). PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta crystallogr. D, Biol. crystallogr. 66, 213-21. 7. Smith, D. K., Radivojac, P., Obradovic, Z., Dunker, A. K. & Zhu, G. (2003). Improved amino acid flexibility parameters. Prot. Aci. 12, 1060-72. 8. Bertini, I., Fragai, M., Luchinat, C. & Parigi, G. (2000). 1H NMRD profiles of diamagnetic proteins: a model-free analysis. Magn. Reson. Chem. 38, 543-550.