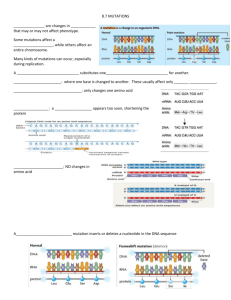
Complementation of lethal mutations
advertisement

Fly Genetics (fall 2011) Pat O’Farrell ofarrell@cgl.ucsf.edu - 6-4707 Lecture 2 Screens & genetics of development Genetics in this lecture: • A synergy of the biology of Drosophila and its powerful genetics broke open the field of developmental biology. • A game changing genetic screen for embryonic mutations. • Combining molecular and epistasis data to get clear conclusions. • Sensitized backgrounds and a focused approach to genetic screens • Making genetic mosaics using mitotic recombination reveals unexpected lineage barriers, reveals the existence of epigenetics and defines its biological roles. Developmental Principles of building body form: • Cells in different positions do different things. • A grid of localized developmental regulators creates a coordinate system that tells a cell where it is in an embryo. These patterning regulators are conserved among metazoans. • Cells "remember" early embryonic position via a stable epigenetic program that causes cells from different locations to do different things. The genes governing this epigenetic program were defined in flies and are the focus of the biochemistry of chromatin structure. General reading: Eric Wieschaus Nobel Lecture – excellent description of thought behind the extraordinary screen for all of the genes that acted zygotically control embryonic patterning (repeat of lect 1 suggestion). http://nobelprize.org/nobel_prizes/medicine/laureates/1995/wieschaus-lecture.html A paper that has influenced the direct of research in most branches of biology – a real game changer. Nüsslein-Volhard C, Wieschaus E. Mutations affecting segment number and polarity in Drosophila. Nature. 1980 Oct 30;287(5785):795-801 Paper describing the screen and the detailed outcome for chromosome II: Course web site – pdf of Nüsslein-Volhard C, Wieschaus E, Kluding H (1984) Mutations affecting the pattern of the larval cuticle in Drosophila melanogaster. Roux's Archives of Developmental Biology (AKA Development Genes and Evolution) 193, 267-282 Exercise – I do not intend to cover the next three pages of notes in class, and I would like you to work through these notes on your own before the next class. Hopefully you will understand them fully, but if not please bring questions to the next class which will begin by addressing any questions on this material. Please feel free to consult with each other, and the TA's should try their hand at it. Isolating Lethal Mutations and "Cloning" Chromosomes Usually it is hard to isolate something dead, but isolation of lethal mutations is a standard in fly genetics. The facility in doing this is an offshoot of the approach to isolating mutants. To identify mutants in a diploid you have to isolate the genetic contributions of one chromosome – This can be done by removing the contribution of the homolog e.g. by have a homolog with a deletion for the region of interest (we will cover this in an in class problem) or by not having a homolog, as in the X chromosome in males. More generally, the chromosome of interest is made homozygous. In flies, use of balancers allows both identification of homozygotes and retention of mutant chromosomes that carry lethal mutations. Here is how one isolates lethal mutations (an example, other balancers and mutations work). Remember * indicates a collection of mutagenized chromosomes in a population. When a particular mutagenized chromosome is isolated I designate it, usually as *1, but many independently mutagenized chromosomes (different) are in the original pool. The first letter in the names of dominant mutations are UC. Here L designates the Lobe mutation. It is dominant visible (eye shape defect) that is recessive lethal. 1. Mutagenize +/+ and cross to L/SM5 = */L & */SM5 2. Pick one male fly (since you pick one fly for step 2, you are isolating and effectively “cloning an individual * chromosome", here indicated as *1) and cross to fly with marked chromosomes. *1/SM5 X L/SM5 = *1/SM5 & *1/L & L/SM5 3. Pick males and females carrying the cloned candidate chromosome and a Balancer chromosome (e.g. any Cy fly that is not L) and cross these (this is a self cross). *1/SM5 X *1/SM5 = *1/*1 & *1/SM5 & SM5/SM5 not Curly Curly dead NonCurly progeny show that the *1 chromosome is homozygous viable & lacks a lethal, but if all progeny are Curly, the cloned *1 chromosome is homozygous lethal (presumes many progeny). Doing steps 2 and 3 many times (a separate vial for each) clones many * chromosomes and if you discard all vials that yield straight winged flies, you will be left with a bunch of vials each containing a particular * chromosome over a balancer and each of these retained an independently isolated lethal mutation. * chromosomes will carry Note that you mutagenized whole flies, but you specifically collect lethals on the second chromosome. Lethals on other chromosomes will be masked by unmarked homologs and will soon vanish because they are not balanced. Drosophila geneticists use this strategy to “mutagenize one chromosome at a time”, but of course they are not selectively mutagenizing one chromosome at time, but analyzing the mutants one chromosome at a time. Screening for alleles of l(epi) The previously unsolvable part of the class problem #1 in the first lecture Assume that you have a balanced stock of your starting lethal allele (let’s assume that it is on the second chromosome and you use the Curly marked balancer SM5 to keep the stock). l(epi)/SM5 Your problem is to select new alleles of this gene. 1. (like before) Mutagenize +/+ and cross to L/SM5 = */L & */SM5 Pick one male fly (since you pick one fly for step 2, you are isolating and effectively “cloning an individual * chromosome, here indicated as *1) and cross to fly with marked chromosomes. 2. *1/SM5 X l(epi)/SM5 = *1/ l(epi) & *1/SM5 & l(epi)/SM5 & SM5/SM5 not Curly Curly dead If there are nonCurly progeny the *1 chromosome is viable “across from l(epi)” and therefore does not carry a lethal allele of l(epi). Note – when we looked at finding lethals in class, one had to look for crosses with 25% dead flies (really hard); here you just screen for the presence (or absence) of flies with straight wings (incredibly easy). In the screen for general lethals (previous page) you distinguished relevant and non-relevant vials (the ones with straight winged flies) in the progeny of the third cross (an F3 screen), while here, the allele screen allows you to distinguish interesting and irrelevant vials after the second cross (an F2 screen). If you do step 2 many times (using a separate vial each time) you can clone many * chromosomes and if you discard all vials that yield straight winged flies, you will be left with a bunch of vials each containing a particular * chromosome (in trans to the Balancer) that is lethal over l(epi). Assuming there are no recessive lethals other than l(epi) on the chromosome II that carries l(epi) (this is a realistic genetic caution – why?), all remaining * chromosomes will carry a lethal allele of l(epi). How do you retrieve the new mutant and tell it apart from the original l(epi) (ie. distinguish the two types of Curly flies in the progeny of step 2)? You need at least one additional marker in the cross *1/SM5 X l(epi)/SM5. Since the two types of Curly progeny do not have any chromosome in common (by common here I mean derived from the same place/parent, not common as in similar), they can be distinguished by a scorable marker on any of the chromosome IIs in the cross. This is often most conveniently done with an extra marker on the balancer in one of the parents, Here I have assumed that one of the input SM5 balancer chromosomes was tagged with GFP (such GFP marked balancers are common) and I have indicated it in green. Notice that there is an obligate switch in the partnership of the green Balancer and the chromosome being balanced so that before the cross the SM5-GFP was with the starting l(epi) chromosome and after the cross it is with the newly mutant chromosome. To set up a stock of the new mutant, pick the green, Curly flies and simply let them mate (make sure your females are virgin when you set this up!). Remember that the swap of partnerships of chromosomes in a cross exemplified above by the GFP balancer is a general and a reliable feature of Mendelian genetics. Doing a screen Mutagenesis technique: Males are mutagenized because they so fecund that even high doses of mutagen leave adequate fertility. While treatment of sperm can induce DNA damage that is ultimately mutagenic, the damage can persist into the egg and mutations only “fixed” (as in stabilized) in some of the cells of the embryo. To avoid (reduce) such mosaic progeny time is allowed after mutagen treatment to allow damage in sperm progenitor cells to be fixed as mutations and to make their way into mature sperm. If you wait too long, many sperm may derived from a few mutant stem cells and one ends up with “jackpot” in which the same mutant is isolated many times (a bad thing). Allele frequency and mutagen dose: The choice of level of mutation is a matter of balancing practical issues. Too high a dose and you kill everything. Too low a dose and you have to work very hard to get your mutants. But a higher mutation frequency is not always better, because you then have multiple mutations in each progeny and even each chromosome of the progeny. Then you have to work hard to resolve the lesion that is responsible for the phenotype from the background. The allele frequency is the frequency at which you get new alleles of a given gene (or in other words the frequency with which you hit a gene). In flies one often assess the allele frequency by scoring for white eyes in the progeny (F2 males or F1 females after mating with w/w females). A high level EMS mutagenesis will give an allele frequency of about 1/1000. Why would you white eyes in this assay? ignore the males with A screen for mutations on chromosome II (the same as cloning many mutagenized chromosomes – two pages previous) Scoring for the absence of straight winged flies isolates any lethal as covered above. You can score for viable phenotypes in the straight winged flies – for example an eye color mutant like cinnabar would show up. Indeed any recessive mutation giving a scorable defect could be isolated. When there is a specific lethal phenotype is of interest (e.g. larvae that arrest before pupariation with an enlarged red ring gland – just a strange one that we have a current interest in), you can screen for it directly and establish a stock from the surviving heterozygotes. In Class Problem 2: (Taken from real life – see Regulski et al., 2004 and Yakubovich et al., 2010) Nitric oxide (NO) is a very simple but reactive molecule that has been ascribed numerous biological roles (the literature on NO is larger than the literature on yeast). An enzyme that makes NO, called nitric oxide synthase or NOS, is conserved from bacteria to human. You (Regulski) wanted to isolate a mutation in the fly enzyme, whose coding sequence was readily recognizable in the genome sequence at 69F (toward the middle of the right arm of chromosome II). You have at your disposal a deletion (Df (69F)) of a little more than 20 kb of sequence that removes the NOS promoter region and 3 N-terminal exons, as well as five other upstream genes (deleted in whole or in part). At least one of the upstream genes is essential (there are lethal alleles), so this deletion is necessarily also lethal. If you assume (as Regulski did) that a NOS mutation would be lethal, how might you isolate a mutant. Step 1 Mutagenize males. (en mass) Step 2 Cross mutagenized males with second chromosome balancer flies (en mass) and collect progeny that have mutagenized II over balancer (individuals). Choose males (more fecund and easier in the next cross). Step 3 In vials – cross individual males that are */CyO KrGFP with females that are Df(69F)/CyO and score the vials 2 weeks later to determine whether there are straight winged progeny (these are presumed to have good copies of all essential genes within Df(69F) and these must have come from the male). Straight winged flies shows that no lethal has been generated in the region of the deletion and the flies/vial are discarded (there were 4823 of these). The absence of straight winged flies suggests that the combination of */Df(69F) is lethal. 32 lethal lines were recovered. Step 4 Set up stocks of mutant. Collect non GFP Cy flies – these have * chromosome opposite CyO (as opposed to CyO KrGFP-note balancer swap between generations). Either cross with virgins of same or cross to Sp/Cyo and then cross non Sp, Cy progeny inter se. Step 5 – Place lethals into complementation groups – you should be able to describe how this would be done. Table S1. Screen summary. Total lines screened Lethal hits within Df(2L)69F Lethal Complementation Groups within Df(2L)69F: Group 1 Mitochondrial Porin Group 2 Group 3 Group 4 Group 5 4855 32 3 alleles 17 alleles 6 alleles 2 alleles 4 alleles We screened 4855 lines and identified 32 mutations, which were lethal over the Df(2L)69F deficiency. Inter se complementation analysis split them into 5 groups. Group 1 failed to complement the l(2)k08405 P-element insertion and, thus, represents 3 new alleles of the mitochondrial porin gene. Group 2, the largest with 17 alleles, corresponds to dNOS (see below). Inter se complementation between all groups indicated a genetic interaction between Groups 2 and 3. Some heteroallelic combinations result in sterile or almost sterile flies, suggesting that mutations in Groups 2 and 3 may affect the same gene. Groups 4 and 5 do not show any genetic interactions. Step 6 Assay (in their case one mutant) for NOS activity. Find a deficient allele and sequence to show a base change in conserved residue of NOS. They expressed the mutant protein in bacteria and showed that it lacked NOS activity while a wild-type sequence produced NOS activity. They published a paper saying NOS is essential for viability in Drosophila. Did they do enough to justify this? Sensitized Genetics A powerful to screen for genes that contribute to a particular biological function. This approach was first developed and used by Mike Simon, an ex-student of our department, when a post-doc in Gerry Rubin's lab. Simon MA, Bowtell DD, Dodson GS, Laverty TR, Rubin GM (1991) Ras1 and a putative guanine nucleotide exchange factor perform crucial steps in signaling by the sevenless protein tyrosine kinase. Cell 67, 701-16 Idea based on several facts. 1. One good copy of almost any region in the genome is sufficient for wt function (demonstrated by small deletions genome wide Lindsley et al., 1972) (haploinsufficiency is rare) 2. Removing one copy of a gene reduces its function in half (Muller et al., 1931) (Surprising fact that has been verified repeatedly and is true for the vast majority of genes). 3. When an essential pathway has been compromised by a hypomorphic mutation so that a weak phenotype is seen – the severity of this phenotype is sensitive to conditions (opposite to robust). Also, weak ectopic activation of pathways also gives non-robust behavior. It was argued that if the phenotype is very sensitive to the activity of pathway reducing the dose of another gene in the pathway might further reduce the activity of the pathway and exacerbate the phenotype, while the same heterozygous defect would have no effect in the absence of the sensitizing background. The authors studied receptor tyrosine kinases that are required for normal eye development and discovered Ras and Ras regulatory proteins as enhancers of defects in RTK function and suppressors of mild gain-of-function RTK mutant. Their work ended up organizing the previously chaotic field of Ras signaling, and revealed a powerful and general way to screen for genes in particular functional pathway. WT eye Ellipse (gain of function RTK) Ellipse, modifier mutant/+ Almost any process in molecular biology, cell biology, or development can be dissected by modifier screens in the eye (note the eye is best but similar things can be done in other tissues). To do this one might express an RNAi in the eye that compromised a particular process, and then cross to introduce mutagenized chromosomes and identify modifiers of the phenotype. Note that these show up as dominants even if they are effectively recessive genes. As a dominant screen, there is no requirement to homozygous the mutation. Individual F1 progeny are screened and the mutants subsequently recovered. Many of the mutants are recessive lethals – explain this to yourself. Some Concepts Connected to Sensitized Genetics Epistasis: Classical epistasis is based on the rationale that if one mutation breaks a pathway, inactivating a second gene in the pathway will have no consequence. This foundation for epistasis is diametrically opposed to the underlying assumption of sensitized genetics – damaging the pathway makes it sensitive to second change . Which is right? Consider whether the mutations are null and consider whether the idea of a pathway is the same. Characterizing mutations with gene dose: Mutant alleles can be Reduction of function mutations, which can be — nulls — hypomorphs — dominant negative mutations — haploinsufficient Gain of function — hypermorph — neomorph How do you tell them apart? Scoring the phenotype of different genetic combinations. Nature of allele null hypomorph Dom Neg haplo Allele 1/Allele1 Allele 1/null Hypom/hypom Allele 1/hypom Allele 1/Df Df/+ Allele 1/+ Allele 1/+/+ (Dup) ++++ ++++ ++ +++ ++++ Recessive + ++ ++ ++/++ Recessive - - ++++ ++++ ++ ++++ ++++ + +/Dominant +/- ++++ ++++ ++ +++ ++++ + + +/Dominant +/- hypermorph neomorph +++++ + N/A ++ + +++ ++++ Dominant +++++ +++++ N/A +++++ +++++ N/A +++++ +++++ Dominant + Recessive Reversion of dominant mutations is easily identified, is induced by mutagens and is usually due to elimination of function. Classically X-ray induced reversion of dominant alleles was heavily utilized to generate deletions in the region of the dominant mutation. — A Little development Genomic information dictates body structure. But how do genes direct production of large scale form and structure? How is space patterned? How are organs and limbs sized and shaped? In 1979Not a single developmental regulator was known. Two centuries of study of development gave us descriptions of development but not mechanism. Signaling had been documented but no signal identified. A Genetic Challenge Find the genes governing development Remember most of the molecular hardware for metabolism and normal cell function is already deposited in the egg. How many genes would be required to make the patterned embryo that hatches out of the egg case 24 hr later? What would these genes be required for? Would their phenotypes (and eventual molecular characterization tell us anything about development)? Could approach this in comprehensive fashion, and find all of the required genes? Considerations – • Expect the mutations of interest to be lethal, likely embryonic lethal, and not to hatch. • Interesting ones should make a cuticle with defective pattern. • To get mutations in all of the genes that are involved, have to assay enough mutations to assay a hit in every gene of the genome. Practical level – >5 times the allele frequency ~ 99% chance of getting any one gene that is required and has ~ average allele frequency. Doing the Screen – Chromosome II Eric Wieschaus and Christiane Nüsslein-Volhard streamlined procedures to very efficiently identify genes that affected the ability of the embryos to hatch and to screen for defects in the embryonic cuticle. They used a chromosome II Balancer called CyO (atypical name, but typical Balancer), and a dominant temperature sensitive mutation (DTS) to help them get rid of a class of unwanted progeny and thereby save some work. This was their set up (simplified a bit). In the F1, mutagenized chrsomosomes were over either CyO or DTS. Even though the DTS is not balancer they did not worry about recombination at this step. Why? They set up 10,000 of these F1 crosses. They eliminated all the DTS progeny in the F2. If the mutagenized chromosome included a lethal, the only viable progeny would be the newly mutant chromosome over CyO. If straight-winged progeny were included, the cross was eliminated. Embryos homozygous for the Balancer hatch and die as larvae. To find embryonic lethals, they looked at the eggs when they should have hatched (24 h after egg laying). If 25% fail to hatch, there is an embryonic lethal. They then looked at all candidate embryonic lethals (?) for pattern defects. How much they did and Yield: They screened 5,764 candidate mutagenized chromosomes. These included 4,217 chromosomes that included a lethal. Because some chromosomes have more than one lethal hit, this corresponds to 7,600 lethal hits. 25% of lethal lines included an embryonic lethal – i.e. 25% of the embryos did not hatch. 272 mutants (about 3.5% of lethal hits) had visible defects in the cuticular pattern. These fell into 61 complementation groups. Denticles – traction hairs on surface of larvae illustrate pattern Dark field showing belts of ventral denticles as arranged in wild type larva (or mature embryo). These are easily visualized. A detail view showing the finer features of pattern in a denticle belt Dilks and DiNardo These patterns of denticles allowed investigators to distinguish the different segments, and the details of organization within segments. Consequently they were able to identify and distinguish mutants that altered these aspects of embryonic pattern (among others). What they found – zygotic patterning gene phenotypes This is panel of mutant types from the Nature paper (see reading) that helped inform our appreciation of development of pattern. Expression and pattern The genes discovered in the screen are required zygotically and they control development by controlling where and when things happen. The gene products do not possess the spatial and temporal information, rather this is encoded in their program of expression. Thus, the gene products stimulate something to happen and where and when they do this depends on where and when the genes are expressed. Where and when they are expressed turns out to due to the regulatory interactions among the genes themselves – that is the pattern is emergent property of the system. Fortunately, as the genetics suggested the organization of this system is simple in principle even though it is complex in its details. Here is the story Molecular epistasis Principle; Genes don’t act before they are expressed. If one mutation affects the onset of expression of another, it acts upstream of it. Mutations of pair rule genes delete every other segment. fushi tarazu , ftz, deletes odd numbered abdominal segments, and paired, prd, deletes from the middle of an odd numbered segment to the middle of an even numbered segement. If you stain for the engrailed protein whose expression is usually induced in every segment primordial, you find that ftz mutant embryos are missing half the engrailed stripes (called the even numbered stripes just because they are numbered differently than the abdominal segments) and prd embryos are missing the odd numbered engrailed stripes. Engrailed antibody staining wild-type ftz Odd stripes only prd Even stripes only Therefore, ftz and prd function upstream of engrailed. Epistasis can be combined with other information If genes that are required in one tissue are shown to be epistatic on genes that function in a different tissue, you can conclude that one tissue signals the other. The direction of the signaling is suggested by the epistasis and it can be defined conclusively if genes in one tissue can be shown to be required for expression of genes in the other tissue (molecular epistasis). Knowing where genes function is important in developmental biology. Testing function is not the same as testing expression, since a gene product can be present in multiple tissues and only required in one special circumstance. On the other hand genes don’t function where they are not expressed – so there is a connection. How do we determine where genes functions and what do we learn? DiNardo, S. and O'Farrell, P.H. (1987) Establishment and refinement of segmental pattern in the Drosophila embryo: spatial control of engrailed expression by pair-rule genes. Genes and Development 1: 1212-1225. Mosaic analysis Mutations can be made homozygous in the background of normal (heterozygous) fly by the induction of recombination between a centromere and gene of interest. Just marking cells (e.g. with little a versus A) tells us things about lineage and growth during development. Example – The wings of flies have little hairs – one hair/cell that are orderly arranged Several mutations disturb these hairs. For example multiple wing hair (mwh) has multiple and disorderly hairs. Clones homozygous for mwh are readily visible on a background of normal hairs (Fig right: a big clone of mwh/mwh in the anterior part of the wing). When clones are induced by early X-ray treatment, one tends to get few large clones, and when they are induced later they occur at higher frequency but are smaller. Why? Clones reveal whether cell fate is coupled to lineage (stereotyped positions of clones), or not. Though elongated by morphogenic movements, the wing clones are random, except that there is line that they never cross, a “line of clonal restriction”. This line defined two compartments, the anterior and the posterior. This division is of major importance in development. More on Mosaic analysis The Minute technique. The are a number of different genes (~60) with a similar dominant phenotype: they have small (minute) bristles and slow growth. Many of these encode ribosomal proteins and it is thought that they globally reduce protein synthesis when in one copy (haploinsufficient). They are lethal when homozygous. Mitotic recombination of a Minute heterozygote (M/+) will give one sister cell that is wild-type and is referred to as a “Minute plus” or M+ clone, and one sister that is M/M and dies. The surviving M+ clone out grows the surrounding cells and contributes disproportionately to the final structures, but all the pattering is normal. Such M+ clones can fill an entire compartment (at least the surface visible part) indicating that one cell had the potential to produce all the structures and was able to out compete all the other cells. But size is normal and the clone never crosses into other compartments Engrailed expression (left antibody staining of wing disc & right reporter expression in wing) marks the posterior compartment. Twin spot – There are two mutations arranged in trans as are A/a and b/B in the figure (prev. page), recombination can mark both sister cells one as little a and one as little b. If both are visible, one sees a twin spot. This allows one to score what the sister cells do, to determine whether the two sister clones grow at the same rate etc. The two eyes show the twins of induced mitotic recombination. In the lower image the white member of the clone includes a mutation in growth suppressor function so that it out competes the red sister. This scanning EM illustrates the 800 ommatidia of the fly eye. The eye is dispensable and phenotypes representing cell cycle, development, receptor tyrosine kinase signaling, growth, cell patterning, nerve cell function can all be screened and scored in clones.