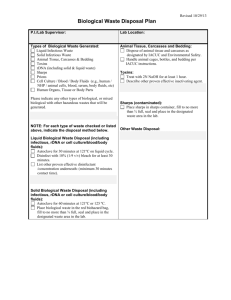
waste v1 - dorsetldc.org - Dorset Local Dental Committee
advertisement

Infection Prevention & Control Policy & Audit Tool POLICY FOR THE MANAGEMENT OF WASTE IN THE COMMUNITY Date of Issue March 2010 Date of Review March 2013 D:\116103232.doc DOCUMENT HISTORY Document Status: Developed by: Current Health Records and Transport Manager, Dorset County Hospital Foundation NHS Trust, Reviewed by Tracey Stevenson for GP, Dentist and Care Home use Policy Number ID, Version 1.1 Date of Policy March 2010 Next Review Date March 2013 Health Records and Transport Manager, Dorset County Hospital Foundation Sponsor NHS Trust Approved by / on Health, Safety and Welfare Group, Dorset County Hospital Foundation NHS Trust Consulted Dorset Infection Control Forum Sept 2010 Version Date 1.1 D:\116103232.doc Comments CONTENTS 1. POLICY STATEMENT .............................................................................................. 1 2. INTRODUCTION....................................................................................................... 1 3. CHANGES INTRODUCED IN THIS GUIDANCE ....................................................... 1 4. LEGISLATION, REGULATIONS AND TRUST POLICIES ......................................... 2 5. CATEGORIES, DEFINITIONS AND SEGREGATION OF WASTE............................ 3 6. HAZARDOUS WASTE .............................................................................................. 7 7. INFECTIOUS WASTES…………………………………………………………………….8 8. ANATOMICAL WASTE BLOOD BAGS AND GEL AGENTS ................................... 11 9. MEDICINAL WASTE ............................................................................................... 12 10. PHARMACEUTICAL WASTE…………………………………………………………….13 11. LIQUID WASTES .................................................................................................... 14 12 TOTAL PARENTERAL NUTRICIAN (TPN) ............................................................. 14 13. AMALGAM HAZARDOUS WASTE ......................................................................... 14 14. MERCURY WASTE ................................................................................................ 15 15. OTHER DEFINITIONS ASSOCIATED WITH HEALTHCARE WASTE .................... 15 16. LARGE EQUIPMENT .............................................................................................. 16 17. HIGH TEMPERATURE PROCESSES (INCINERATION) ........................................ 17 18. ALTERNATIVE TECHNOLOGIES........................................................................... 17 19. LANDFILL ............................................................................................................... 17 20. DISCHARGE TO SEWER ....................................................................................... 17 21. ROLES AND RESPONSIBILITIES .......................................................................... 18 22. STORAGE PRECAUTIONS .................................................................................... 19 23. CLEANING OF BULK TRANSPORT ITEMS ........................................................... 20 24. PERSONAL PROTECTIVE EQUIPMENT ............................................................... 20 25. BASIC HYGIENE .................................................................................................... 20 26. IMMUNISATION...................................................................................................... 20 27. ACCIDENTS AND INCIDENTS ............................................................................... 21 28. REPORTING OF INJURIES, DISEASES AND DANGEROUS OCCURRENCES REGULATIONS (RIDDOR) ..................................................................................... 21 29. TRAINING ………………………………………………………………………………….22 29. AUDIT ..................................................................................................................... 22 APPENDIX A WASTE SEGREGATION CHART ................................................................ 26 APPENDIX B EUROPEAN WASTE CATALOGUE CHAPTERS ......................................... 27 D:\116103232.doc APPENDIX C CYTOTOXIC AND CYTOSTATIC MEDICINES ........................................... 28 APPENDIX D COMMUNITY CARE …… …………………………………………….31 D:\116103232.doc MANAGEMENT OF CLINICAL WASTE POLICY 1. POLICY STATEMENT 1.1 The management of healthcare waste is essential to ensure healthcare activities do not pose a risk of infection as outlined in the Health and Social Care Act 2008. 1.2 Care will be delivered without discrimination, regardless of gender/ transgender, race, disability, sexual orientation, age, religion/belief or cultural practice. 1.3 Information will be given to all clients in a way in which they can understand it. 2. INTRODUCTION 2.1 The Clinical Waste Management Policy provides guidance on the safe management and disposal of all types of waste generated within the premises. The Policy specifies how waste should be segregated, stored, handled, transported and disposed of safely and efficiently. This is to reduce the risk of exposure to clients, staff, visitors, refuse collectors and the general public. 3. CHANGES INTRODUCED IN THIS GUIDANCE/POLICY 3.1 This policy utilises the guidance available in “Environment & Sustainability, Health Technical Memorandum 07-01: Safe management of Healthcare Waste” which was produced by the Department of Health in 2006 and is in line with the use of the European Waste Catalogue (EWC) Codes which are now mandatory for all waste transfer documentation The biggest change between this and previous policies is the move away from the use of waste categories A to E as it is felt that these no longer appropriately reflect the segregation of waste for treatment or disposal and do not easily equate to the. Does this above need to be in? 3.2 The policy outlines new methodology for identifying and classifying infectious and medicinal wastes that complies with health and safety, carriage and waste regulations. Compliance with the unified approach will ensure that producers comply with and go beyond the regulatory requirements. 3.3 Changes detailed within the new policy include: The definition and classification of infectious wastes in accordance with hazardous waste regulation and associated guidance published by the regulatory agencies; The definition and classification of medicinal wastes, including cytotoxic and cytostatic wastes, in accordance with hazardous waste regulation and associated guidance published by the regulatory agencies; Changes in carriage regulations brought in by the Carriage Regulations, as amended in 2005; 1 D:\116103232.doc A revised colour coded best practice waste segregation and packaging system; The use of European Waste Catalogue (EWC) Codes. 4. LEGISLATION, REGULATIONS AND POLICIES 4.1 This Policy is designed to confirm safe handling, segregation, packaging and disposal of waste laid down in the: Manual Handling Operations Regulations 1992 Personal Protective Equipment at work Regulations 1992 Health and Safety at Work Act 1974 Control of Substances Hazardous to Health Regulations 2002 Environmental Protection Act (EPA) 1990 (and subsequent amendments) Radioactive Substances Act 1993 Safe Disposal of Clinical Waste, Health and Safety Executive 1999 Labelling) and Use of Transportable Pressure Receptacles Regulations 1999 – modified 2005 - Delivering a Quality Service 1996 and associated regulations 4.2 Hazardous Waste Regulations (2005) European Waste Catalogue Environmental Protection (Duty of Care) Regulations 1991 EC Directive on Waste Electrical and Electronic Equipment (WEEE) and EC Directive on the Restriction of the Use of Certain Hazardous Substances in Electrical and Electronic Equipment (RoHS) – both of which will be phased in during 2005/2006 Waste Management Licensing Regulations; Landfill Regulations; Waste Electric and Electronic Equipment Regulations; The Manager will assess their compliance with this legislation through regular review. 2 D:\116103232.doc 4.3 Other sources of further information include: The Management of Health and Safety in the Health Services, produced by the Health Service Advisory Committee (HSAC) Biological Agents: Managing the Risk in Laboratories and Health Care Premises, produced by the Advisory Committee on Dangerous Pathogens and published on the Health and Safety Website (HSE). Working with ADR – An introduction to the Carriage of Dangerous Goods by Road, produced by the HSE and available on their website. 4.4 A full list of all current guidance can be found at the end of the Environment and Sustainability, Health Technical Memorandum 07-01: Safe management of healthcare waste which can be found at: http://www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAnd Guidance/DH_063274 4.5 DUTY OF CARE 4.6 One of the most far-reaching implications set out by the Environmental Protection Act is the Duty of Care, (Section 34). The Duty of Care requires that as waste producers, the premises ensure waste is not illegally disposed of, does not escape from a person’s control, and is only transferred, with a transfer note, to an authorised person4.7 In addition, Section 3, sub-section 1 of the Health and Safety at Work Act, 1974 states that employers have “a duty to ensure, so far as is reasonably practicable, that persons not in his employment who may be affected are not thereby exposed to risks to their health and safety”. This is the piece of legislation used to prosecute those who have endangered members of the public through poor methods of clinical waste management. 5. CATEGORIES, DEFINITIONS AND SEGREGATION OF WASTE 5.1 This section outlines the definitions and classifications used for healthcare waste in the UK, Carriage (transport) and Health and Safety legislation. Waste regulation requires the classification of waste on the basis of hazardous characteristics and point of production. 5.2 For ease of use, this policy aims to cover within each subsection, the legal definition, segregation, colour coding, packaging and disposal method applicable in each case. In order to do so the policy will refer to the page in what? for the appropriate table or diagram sited within this policy. 5.3 DEFINITIONS AND CLASSIFICATIONS FOR HEALTH AND SAFETY 5.4 Health and safety legislation does not contain any specific waste definitions or classifications. However, the regulations (notably COSHH) require those dealing with hazardous and potentially infectious materials, (including waste), to assess the risk to both their staff and the public who may come into contact with the material. COSHH specifically requires consideration of the biological agents that may be present and hazard groups they belong to. Reference should be made to the COSHH Approved Code of Practice and the Approved List of Biological Agents. Each premise will have a COSHH lead that will be able to provide assistance in this matter. 3 D:\116103232.doc 5.5 NATIONAL COLOUR-CODING APPROACH 5.6 Segregation of waste at the point of production into suitable colour-coded packaging is vital to good waste management. Health and safety, carriage and waste regulations require that waste is handled, transported and disposed of in a safe and effective manner. The following colour-coded waste segregation guides represents best practice and ensures, at minimum, compliance with current regulation. For an overall picture please refer to the diagram in Appendix A 5.7 RECYCLABLE WASTE 5.8 To comply with environmental policies it is encouraged that all recyclable items are separated i.e. plastic cups, cans and tins, glass, newspapers and magazines, clean white used office paper (not confidential) and cardboard should be separated and place in the designated containers for collection. 5.9 Refrigerators/Freezers, Fluorescent Tubes and Batteries. Advice needs to be sought on the disposal of these products. 5.10 Foil and Printer Cartridges. Where possible these should be recycled. 5.11 COMPUTERS AND EQUIPMENT 5.12 When considering disposal of this equipment consideration should be given to see if it can be repaired, re-used or recycled. 5.13 GENERAL WASTE DISPOSAL 5.14 General waste from offices and kitchens must be disposed of in a black plastic bag. Each bag must be appropriately tied and will be collected by a local contractor to remove it from the premises for disposal. This waste MUST be stored separately from Clinical Waste. 5.15 CONFIDENTIAL WASTE 5.16 All employees are responsible for maintaining the confidentiality of information gained during their employment. 5.17 Confidential information can be anything that relates to clients, staff (including noncontract, volunteers, bank and agency staff, locums, student placements) their family or friends, however stored. 5.18 Confidential waste can take many forms including medical notes, audits, employee records, occupational health records etc. It also includes company confidential information. 5.19 Person identifiable information is anything that contains the means to identify a person e.g. name, address, postcode (part or full), date of birth, NHS national insurance number, even a visual image (photograph) is sufficient to identify an individual. 5.20 Certain categories of information are legally defined as particularly sensitive and should be most carefully protected by additional requirements. For example information regarding sexually transmitted diseases, HIV etc. 4 D:\116103232.doc 5.21 Confidential Waste should be placed in plastic locked containers provided by the contractor or in green bags, which should be securely tied, which will be disposed of by shredding. 5.22 ELECTRICAL WASTE 5.23 The majority of electrical equipment will now be classed as Electrical Electronic Equipment (EEE) as defined by the Waste Electrical and Electronic Equipment Directive (WEEE). All WEEE is defined as hazardous waste and should be disposed of in an appropriate manner. 5.24 SPECIAL WASTE (HAZARDOUS WASTE) 5.25 Any waste that presents a risk of death or serious tissue damage following exposure, e.g. radioactive and/or cytotoxic waste is classed as Special Waste. Some clinical waste is also classified as ‘special waste’ e.g. prescription only medicines and syringes contaminated with or still containing prescription only medicines. 5.26 CLINICAL WASTE 5.27 The definition of clinical waste has historically been used to describe those wastes produced from healthcare and similar activities that pose a risk of infection or may prove hazardous. Clinical waste should be segregated from other wastes and treated/disposed of appropriately in suitably licensed facilities on the basis of the hazard it poses. 5.28 The current legal definition of clinical waste in the UK is taken from The Controlled Waste Regulations 1992, issued under the Environment Protection Act 1990. It has remained unchanged since it was first issued under the Collection and Disposal of Waste Regulations 1988, issued pursuant to the Control of Pollution Act 1974. 5.29 Clinical Waste is defined as: “… any waste which consists wholly or partly of human or animal tissue, blood or other bodily fluids, excretions, drugs or other pharmaceutical products, swabs or dressings, syringes, needles or other sharp instruments, being waste which unless rendered safe may prove hazardous to any person coming into contact with it; and any other waste arising from medical, nursing, dental, veterinary, pharmaceutical or similar practice, investigation, treatment, care, teaching or research, or the collection of blood for transfusion, being waste which may cause infection to any person coming into contact with it.” 5.30 EUROPEAN WASTE CATALOGUE 5.31 Recent regulatory changes, notably the Landfill (England and Wales) Regulations 2002, The Hazardous Waste (England and Wales) Regulations 2005 and the List of Waste (England) Regulations 2005, require producers to adequately describe their waste using both a written description and the use of the appropriate European Waste Catalogue (EWC) Code(s). 5.32 The EWC is a list of wastes produced by the European Commission in accordance with the European Waste Framework Directive (75/442/EEC) to provide common terminology for describing waste throughout Europe. The EWC list is reviewed 5 D:\116103232.doc periodically and incorporates the European Hazardous Waste List pursuant to the Hazardous Waste Directive 91/689/Eec. 5.33 The EWC is colour coded to aid identification of hazardous waste. Absolute entries* (shown in red) in the catalogue are deemed to be hazardous regardless of their composition or concentration. Mirror entries (shown in blue) are those, which are recognised as having the potential to be hazardous and require an assessment of their composition and concentration. Non-hazardous wastes are shown in black. The EWC categorises waste into 20 chapters; each chapter is linked to a production sector. 5.34 Healthcare wastes are listed in chapter 18 of the EWC, however, producers should be aware that healthcare premises produce a wide variety of wastes and reference should be made to other relevant EWC chapters (the full waste catalogue chapters can be found in appendix B). 5.35 Within each chapter wastes are described using 6 digit numerical codes, the first two digits of the code relate to the EWC chapter, the second two digits relate to any subgrouping within the chapter and the final two digits are unique to the waste. 5.36 The table below provides a list of all Chapter 18 (Healthcare Waste) EWC Codes. 18 01 02 (black) body parts and organs including blood bags and blood preserves (except 18 01 03) 18 01 03* (red) wastes whose collection and disposal is subject to special requirements in order to prevent infection 18 01 04 (black) wastes whose collection and disposal is not subject to special requirements in order to prevent infection (for example dressings, plaster casts, linen, disposable clothing, diapers) 18 01 06* (blue) chemicals consisting of or containing dangerous substances 18 01 07 (black) chemicals other than those mentioned in 18 01 06 18 01 08* (red) cytotoxic and cytostatic medicines 18 01 09 (black) medicines other than those mentioned in 18 01 08 18 01 10* (red) amalgam waste from dental care * Hazardous Waste List Entries 5.37 The use of the EWC has led to a change in the classification of infectious and medicinal waste in the UK. A number of entries in chapter 18 of the EWC are classified as hazardous waste. 6 D:\116103232.doc 6. HAZARDOUS WASTE 6.1 The Hazardous Waste (England and Wales) Regulations 2005 and the List of Waste (England) Regulations 2005 define and regulate the management of hazardous waste in England. These Regulations, amongst other things, require producers of hazardous waste to notify (register) with the Environment Agency. The Regulations do not provide comprehensive guidance on the classification of waste. However, guidance is provided by the UK environmental regulatory authorities (WM2). 6.2 HAZARDOUS WASTE GUIDANCE WM2 6.3 The UK environmental agencies, i.e. the Environment Agency (EA), Scottish Environment Protection Agency (SEPA), have produced a joint guidance document on interpretation, definition and classification of hazardous waste, titled: WM2. This document is available on their website: www.environmentagency.gov.uk/commondata/acrobat/1_haz_waste_intro.pdf. 6.4 WM2 provides guidance on the classification of absolute* and mirror entries in the EWC in relation to the 14 hazard groups identified in the Hazardous Waste Regulations 2005. The 14 hazard groups originate from the Hazardous Waste Directive and are shown below. H1 “Explosive”: substances and preparations which may explode under the effect of flame or which are more sensitive to shocks or friction than dinitrobenzene. H2 “Oxidising”: substances and preparations, which exhibit highly exothermic reactions when in contact with other substances, particularly flammable substances. H3A “Highly Flammable” - liquid substances and preparations having a flashpoint of below 21°C (including extremely flammable liquids), or - substances and preparations which may become hot and finally catch fire in contact with air at ambient temperature without any application of energy, or - solid substances and preparations which may readily catch fire after brief contact with a source of ignition and which continue to burn or to be consumed after removal of the source of ignition, or - gaseous substances and preparations which are flammable in air at normal pressure, or - substances and preparations, which, in contact with water or damp air, evolve highly flammable gases in dangerous quantities. H3B “Flammable”: liquid substances and preparations having a flashpoint equal to or greater than 21°C and less than or equal to 55°C. H4 “Irritant”: non-corrosive substances and preparations, which, through immediate, prolonged or repeated contact with the skin or mucous membrane, can cause inflammation. 7 D:\116103232.doc H5 “Harmful”: substances and preparations which, if they are inhaled or ingested or if they penetrate the skin, may involve limited health risks. H6 “Toxic”: substances and preparations (including very toxic substances and preparations) which, if they are inhaled or ingested or if they penetrate the skin, may involve serious, acute or chronic health risks and even death. H7 “Carcinogenic”: substances and preparations which, if they are inhaled or ingested or if they penetrate the skin, may induce cancer or increase its incidence. H8 “Corrosive”: substances and preparations, which may destroy living tissue on contact. H9 “Infectious”: substances containing viable microorganisms or their toxins, which are known or reliably believed to cause disease in man or other living organisms. H10(2) “Toxic for reproduction”: substances and preparations which, if they are inhaled or ingested or if they penetrate the skin, may produce or increase the incidence of non-heritable adverse effects in the progeny and/or of male or female reproductive functions or capacity. H11 “Mutagenic”: substances and preparations which, if they are inhaled or ingested or if they penetrate the skin, may induce hereditary genetic defects or increase their incidence. H12 Substances and preparations, which release toxic or very toxic gases in contact with water, air or an acid. H13 Substances and preparations capable by any means, after disposal, of yielding another substance, e.g. a leachate, which possesses any of the characteristics listed above. H14 “Ecotoxic”: substances and preparations, which present or may present immediate or delayed risks for one or more sectors of the environment. 6.5 EWC 2002 states that “Toxic for reproduction” is considered to be in line with the hazardous property H10 “Teratogenic” in the Hazardous Waste Directive. 6.6 Appendix C of the WM2 guidance provides comprehensive guidance on the classification of waste in each of the hazard groups. The following sections provide a summary of the WM2 guidance with respect to infectious, medicinal and amalgam healthcare wastes. 7. INFECTIOUS WASTES 7.1 The Hazardous Waste Regulations 2005 define infectious as: H9 “Infectious”: substances containing viable microorganisms or their toxins, which are known or reliably believed to cause disease in man or other living organisms. 8 D:\116103232.doc 7.2 The WM2 provides additional guidance on the interpretation of this definition in the UK by reference to the need for specialist treatment of disposal, described in WM2 as ‘special requirements in order to prevent infection’ 7.3 Waste defined, as clinical waste on the basis of the infection risk posed should be considered hazardous infectious waste, as the waste requires specialist treatment/disposal. 7.4 The relevant EWC codes is shown below: 18 01 03* (red) wastes whose collection and disposal is subject to special requirements in order to prevent infection 7.5 Identification of infectious waste 7.6 Infectious waste is classified as waste, which may pose a risk of infection to a human or animal. The classification of infectious waste does not rely on the use of pathogen classification groups, and waste should be considered infectious even if the resulting infection would be considered “minor”. 7.7 In order to help clinicians and carers identify potentially infectious waste at the point of production, a simplified assessment has been introduced (see Table 1). The assessment should be used to help staff undertake risk assessments and has not been designed to replace risk assessment or clinical judgement. 9 D:\116103232.doc Table 1 Examples of infectious waste generated as a result of healthcare activities Source General principles Healthcare premises (hospitals, veterinary practices, dentists, nursing homes) Special requirements apply (hazardous by H9) Clinical (or animal healthcare) waste which has not been subject to specific assessment and segregation protocols to remove waste subject to special requirements. The specifically segregated “special requirements” fraction Clinical (or animal healthcare) waste arising from a patient clinically assessed or known to have a disease caused by a micro organism or its toxin, where the causal pathogen or toxin is present in the waste. For example: waste from infectious disease cases; waste from wound infections and other healthcare-associated infections; hygiene products from patients in with UTI infections; waste from patients with diarrhoea and vomiting caused by infectious agents or toxins, for example Clostridium difficile; blood-contaminated dressings from a patient with HIV, hepatitis B, rubella, measles, mumps, influenza or other infection that may be present in the blood; respiratory materials from patients with Pulmonary tuberculosis, influenza, RSV or other respiratory infections; contaminated waste from provision of general healthcare to patients with known or suspected underlying or secondary microbial diseases Special requirements do NOT apply Non-clinical healthcare waste where the “special requirements” fraction has been removed following item and/or patient specific assessment and segregation Non-clinical healthcare waste where the “special requirements” fraction has been removed following item and/or patient specific assessment and segregation Source: WM2 7.8 In general, only waste generated from healthcare practice undertaken by a suitably qualified healthcare practitioner will be considered as infectious waste. Waste from domestic minor first-aid and self-care, of a type that does not involve recourse to a healthcare practitioner, is not included within the scope of this assessment. Similar municipal-type waste from industrial and commercial premises is also excluded. Therefore, soiled waste such as sanitary products and plasters are not considered to be infectious unless a healthcare practitioner gives specific advice to the contrary. 10 D:\116103232.doc 7.9 Colour Coding for Disposal and Disposal Method 7.10 Yellow Infectious Waste which is to be disposed of through incineration (to be consigned as 18 01 03*/09) Minimum treatment/disposal required is incineration in a suitably licensed or permitted facility. Yellow-lidded UN approved solid containers for anatomical waste and blood products and gel agents. Yellow-lidded sharps bins for sharps contaminated with substances, which may carry a risk of infection. Orange Infectious Waste which can be ‘rendered safe’ Minimum treatment/disposal required is to be ‘rendered safe’ in a suitably licensed or permitted facility. Orange bags for ‘soft’ clinical waste such as gloves, aprons, hand towels. 8. ANATOMICAL WASTE BLOOD BAGS AND GEL AGENTS 8.1 For the purpose of this guidance document, the definition of anatomical waste includes body parts or other recognisable anatomical items, which may be offensive to those who come into contact with it. 8.2 The EWC lists anatomical waste with blood bags and blood preserves as shown below: 8.3 18 01 02 (black) body parts and organs including blood bags and blood preserves (except 18 01 03) 18 01 03* (red) wastes whose collection and disposal is subject to special requirements in order to prevent infection Colour Coding for Disposal and Disposal Method Yellow Anatomical waste may often be classified as infectious waste due to its contamination with potentially infectious bodily fluids. This Policy states that all anatomical waste including blood products MUST be disposed of in yellow sealed containers, as this will ensure that they are disposed of by incineration. If pre-wrapped in a clinical waste bag, prior to being placed in the sealed unit, then this bag MUST also be yellow. 8.4 TEETH 8.5 It is recognised that the disposal of teeth is unlikely to cause offence and therefore Dental Practitioners may treat this as a non-anatomical infectious waste. Dental Practitioners must ensure that all wastes are treated appropriately and teeth containing amalgam should be segregated and sent for appropriate recovery/disposal (see http://www.defra.gov.uk/environment/waste/special/ index.htm). 11 D:\116103232.doc 9. MEDICINAL WASTE 9.1 The EWC has entries for medicinal wastes are shown below: 9.2 18 01 08* (red) cytotoxic and cytostatic medicines 18 01 09 (black) medicines other than those mentioned in 18 01 08 Medicinal wastes are classified into two categories: Cytotoxic and cytostatic medicines; Medicines other than those classified as cytotoxic and cytostatic. 9.3 Only cytotoxic and cytostatic medicines are classified as hazardous waste. However, other (non-cyto) medicinal waste may require specialist treatment/disposal. Non-cytomedicinal waste should still be assessed for the other hazardous properties that it may possess e.g. H3B Flammable, H4 Irritant, H5 Harmful or H14 Ecotoxic. 9.4 Definition of Medicinal Wastes 9.5 Medicinal waste includes expired, unused, spilt, and contaminated pharmaceutical products, drugs, vaccines, and sera that are no longer required and need to be disposed of appropriately. The category also includes discarded items used in the handling of pharmaceuticals, such as bottles or boxes with residues, gloves, masks, connecting tubing, syringe bodies and drug vials. There are a number of licensed medicinal products that are not pharmaceutically active and possess no hazardous properties (examples include saline and glucose). These wastes are not considered to be pharmaceutical/medicinal waste for the purposes of this document. 9.6 Only cytotoxic and cytostatic medicines are considered to be hazardous waste. A cytotoxic and cytostatic medicine is a medicinal product possessing any one or more of the stated hazardous properties shown below: H6 Toxic H7 Carcinogenic H10 Mutagenic 9.7 Remember: Non-cyto-medicinal waste should still be assessed for the other hazardous properties that it may possess e.g. H3B Flammable, H4 Irritant, H5 Harmful or H14 Ecotoxic. This assessment is determined solely by assessment of the medicinal products in the form supplied by the manufacturer or distributor, and does not therefore consider the effects of any subsequent dilution that may occur during routine use. Further guidance on the assessment of these hazardous properties may be obtained from Hazardous Waste guidance: WM2. 9.8 Guidance should be sought from the manufacturers of medicinal products with regard to their hazard characteristics. Material safety data sheets (MSDS) (sometimes referred to as COSHH sheets or other product information in local pharmacy practices) may be used to classify products. 12 D:\116103232.doc 9.9 Colour Coding for Disposal and Disposal Method 9.10 Any individual patient medicine, other than Controlled Drugs, no longer required should be returned to a Pharmacy in a secured container. The instructions given below are for the disposal at premises level for sharps containing medicines and used ampoules. Cytotoxic/Cytostatic Waste (see Appendix C for a list of Cytotoxic and Cytostatic medicines, See also British National Formulary chapter 8) Purple with Yellow Yellow Minimum treatment/disposal required is incineration Medicinal Waste This waste is disposed of using yellow-lidded UN approved containers. Minimum treatment/disposal required is incineration. This waste will be consigned as 18 01 03* /09 (red/black) to indicate that sharps containing both medicines and possibly infectious substances (both hazardous in nature) may be within the same container. Yellow Residual Medicinal Waste Residual medicinal waste is waste pharmaceuticals no longer in their original packaging. The waste should be placed in UN-compliant packages for disposal by incineration. If cytotoxic/cytostatic medicinal residues are present, the container should be purple-lidded. 10.0 PHARMACEUTICAL WASTE 10.1 Pharmaceutical waste (or “Medicinal Waste”) includes expired, unused, spilt and contaminated products, drugs, vaccines and sera that are no longer required and need to be disposed of appropriately. 10.2 A risk assessment should be carried out in connection with the drug products and also the act of discarding medicines on site. 10.3 For personal protection whilst disposing of pharmaceuticals, wear latex gloves and apron during the process of sorting and disposing of waste. Basic personal hygiene e.g. hand washing, is also important in reducing the risk from waste. Staff safety is paramount and where it is unsafe or not possible to segregate pharmaceutical waste it should all be consigned as hazardous waste. 10.4 Any outer inert packaging and Patient Information Leaflets may be placed into ordinary paper/cardboard waste containers for recycling; however Patient-Sensitive Information must be obliterated with permanent black pen before disposal. It should be noted that there is no obligation to remove the cardboard outer packaging for recycling but that doing so will reduce the volume of pharmaceutical waste. 10.5 The Environment Agency has confirmed that it supports the removal of a blister strip from other outer packaging, so that the blister strip can be placed in the waste container and the outer original packaging can be recycled. 13 D:\116103232.doc 10.6 DISPOSAL OF PHARMACEUTICAL WASTE IN DOMICILLARY SETTINGS 10.7 Patients or their relatives should be encouraged to return unwanted medication to a community Pharmacy (or Dispensing Practice) for safe disposal. The PCT contracts with a waste carrier to make regular collections of pharmaceutical waste from these settings and the pharmacy contract and Dispensing Services Quality Scheme include mechanisms for checking that such waste is correctly handled. The waste service provides separate bins for the disposal of hazardous waste and it is responsibility of the Pharmacy or dispensing practice to ensure that appropriate segregation of hazardous waste occurs. 10.8 Patient returned medication should not be accepted by GP practices or other community based clinics. It should all be referred to community pharmacies (or dispensing practices) 10.9 Community Pharmacies cannot accept sharps waste (or other as part of an Enhanced Service Needle Exchange Programme). When a patient is prescribed injectable medication or administration in their own home, the GP should prescribe a sharps bin on an FP10 prescription. Sharps bins filled to the recommended level should be sealed and returned to the GP practice for safe disposal. 10.10 It is the responsibility of the General Practices to ensure safe disposal of sharps waste returned to their premises. 10.11 Pharmaceutical waste generated by a clinic GP practice or other healthcare organisation such as home care with nursing must be disposed by that organisation following national policy and cannot be accepted by community pharmacies. 10.12 DISPOSAL OF CONTROLLED DRUGS IN DOMICILLARY SETTINGS 10.13 Patient or their relatives should be encouraged to return any unwanted or unused controlled drugs to their Community Pharmacy (or Dispensing Practice) where appropriate record keeping and disposal facilities will be available. 10.14 All other community based settings where controlled drugs are held will require witnessed destruction of controlled drugs as authorised by the Accountable Officer. 11. LIQUID WASTES 11.1 Liquid waste or solidified liquid waste should be placed in a UN approved rigid leakproof yellow container for disposal. Under Landfill Regulations liquid waste cannot be sent for disposal to a landfill site. 12 TOTAL PARENTERAL NUTRICIAN (TPN) 12.1 Bags containing nutritional products can be discharged to sewer. The emptied bags can then be placed into the domestic waste stream (black bags). 13. AMALGAM HAZARDOUS WASTE 13.1 The only entry for amalgam waste in the EWC is: 18 01 10* (red) amalgam waste from dental care 14 D:\116103232.doc 13.2 Colour Coding for Disposal and Disposal Method 13.3 Amalgam waste consists of amalgam in any form and includes all other materials contaminated with amalgam. Amalgam waste should be placed in white rigid containers with a mercury suppressant. Amalgam waste should be sent to suitable licensed or permitted waste management facilities where the waste undergoes a mercury recovery process prior to final disposal. Amalgam Waste White For recovery 14. MERCURY WASTE 14.1 All waste materials containing or contaminated with mercury are classified as hazardous waste. 14.2 Colour Coding for Disposal and Disposal Method 14.3 There is no colour code for this waste stream. Items known to contain mercury to MUST be safely disposed of by a properly licensed company. 15. OTHER DEFINITIONS ASSOCIATED WITH HEALTHCARE WASTE 15.1 MAGGOTS (Larvae) 15.2 All maggots used for wound management must be secured in an airtight rigid container and marked ‘for incineration’. It is recommended that this should be a sealable UN approved yellow-lidded container. 15.3 MEDICAL DEVICES 15.4 Medical devices are defined in the Medical Devices Regulations as: "An instrument, apparatus, appliance, material or other article, whether used alone or in combination, together with any software necessary for its proper application, which: (a) is intended by the manufacturer to be used for human beings for the purpose of: (i) diagnosis, prevention, monitoring, treatment or alleviation of disease, (ii) diagnosis, monitoring, treatment, alleviation of or compensation for an injury or handicap (iii) investigation, replacement or modification of the anatomy or of a physiological process, or (iv) control of conception; and 15 D:\116103232.doc (b) does not achieve its principal intended action in or on the human body by pharmacological, immunological or metabolic means, even if it is assisted in its function by such means, and includes devices intended to administer a medicinal product or which incorporate as an integral part a substance which, if used separately, would be a medicinal product and which is liable to act on the body with action ancillary to that of the device.” 15.5 Infected/used medical devices 15.6 Where implanted medical devices have been in contact with bodily fluids and have been assessed to be infectious, they should be classified and treated as infectious waste. 15.7 If the device contains hazardous materials or components including nickel cadmium and mercury-containing batteries, the description of the waste on the consignment note must fully describe the waste and all its hazards. For example, an implanted device with a nickel cadmium battery should be classified as: 18 01 03* (red) Infectious waste containing Nickel Cadmium batteries [Hazards: Infectious (H9) and Corrosive (H8)] 15.8 The waste description should accurately describe the waste. 15.9 Disinfected/Unused Medical Devices 15.10 Disinfected medical devices should be classified as non-infectious healthcare waste. The description given of the waste must adequately describe the waste and any hazardous characteristics (even if the waste is not classed as hazardous waste). 15.11 For example a disinfected device containing a nickel cadmium battery should be classified as: 16 02 13 (black) Discarded equipment containing hazardous components other than those mentioned in 16 02 09 to 16 02 12. 15.12 The waste description should accurately describe the waste. 15.13 Other classifications within subchapter 16 02 may apply to disinfected electrical devices. 16. LARGE EQUIPMENT 16.1 Where practicable, equipment should be decontaminated prior to disposal. Once decontaminated, the waste is not subject to carriage or hazardous waste management controls, however, it is still subject to the duty of care. 16.2 Where decontamination is not practicable, producers should contact their waste management contractor to establish the best practice packaging and treatment/disposal options. 16.3 Disposal of large electronic equipment will need to be in accordance with the Waste Electrical and Electronic Equipment Regulations and, if hazardous, the Hazardous Waste Regulations. 16 D:\116103232.doc 16.4 Colour Coding for Disposal and Disposal Method 16.5 There is no colour code for this waste stream. 17. HIGH TEMPERATURE PROCESSES (INCINERATION) 17.1 Pyrolysis 17.2 Pyrolysis involves the high temperature (545 to 1000°C) combustion of waste in the absence of oxygen. In generating these high temperatures, the systems treat, destroy, and reduce the volume of clinical waste. 17.3 Gasification 17.4 Gasification is similar to the process of controlled air incineration in that the waste materials are thermally decomposed, but in an oxygen-starved (sub-stoichiometric) atmosphere. The waste in the gasification process is ignited and reduced in a selfsustaining process. No support fuel is consumed save for that required to initiate combustion. The decomposition results in the generation of volatile gaseous material and, depending on the waste content, various vaporised tar oil fractions. The waste gas is passed through a serious of scrubbers/filters and cyclonic separators to provide a clean “producer gas”. 18. ALTERNATIVE TECHNOLOGIES 18.1 Heat (thermal) disinfection systems – Autoclaves. In autoclaving, saturated steam (steam holding water as a vapour) is introduced into a vessel. 19. LANDFILL 19.1 Once the soft waste has been treated by autoclave it will ultimately be disposed of in deep landfill. 20. DISCHARGE TO SEWER 20.1 Any discharge to sewer, other than domestic sewage, must have the prior agreement of the statutory responsible bodies. Anybody intending to dispose to sewer any waste that may present a substantially greater risk than domestic sewage (such as disposable items that are macerated) should first seek advice. 20.2 Known issues with regard to discharges are: bodily fluids – blood and similar substances, for example from suction canisters or wound drains, should not be discharged to foul sewer without disinfection; photochemicals (X-ray) – these are suitable for recycling, it is poor practice, even if permitted by a discharge consent, to discharge this material to foul sewer; cardboard bed-pans and urine bottles – maceration and discharge of shredded material to foul sewer is known to cause obstruction of the sewage network. It is essential that the sewerage undertaker is aware of the presence 17 D:\116103232.doc of this material, and that it is permitted by the producer’s trade effluent consent. 20.3 Radioactive waste may in certain circumstances (where the Certificate of Authorisation for the Accumulation and Disposal of Radioactive Waste under RSA 93 permits) be disposed of to sewer. 21. ROLES AND RESPONSIBILITIES 22 Managers 22.1 Managers have the ultimate responsibility for all aspects of waste management and will ensure that an appropriate policy and procedure is in place for the discharge of waste management requirements. 22.2 Managers have a responsibility to ensure that adequate training with regard to Waste Disposal has been given to staff and ensuring that any appropriate safe handling equipment is made available. 22.3 Managers are required to arrange collection of clinical waste from the site waste station by a reputable company who can supply full accreditation by the Environment Agency for the tasks they carry out on behalf of this organisation; 22.4 To ensure prior to disposal, the waste is stored in a safe and secure manner. 22.5 To ensure that the waste station is kept clean at all times. 22.6 To ensure that clinical waste is disposed of by the appropriate method, with due care and with the Authorisation conditions of the Environmental Protection Act 1990. 22.7 To ensure that security arrangements of clinical waste are maintained throughout the practise. This includes ensuring that all clinical Waste bins are locked at all times. 22.8 Staff 22.9 All staff who may be required to move bags of clinical waste by hand within a particular location should be trained to: a) Check that the storage bags are effectively sealed and the origin of waste clearly identified b) Handle the bags by the neck only c) Know the procedure in the case of accidental spillage and to report the incident using the appropriate Risk Event Form d) Check that the seal of any storage bag is unbroken when movement is complete e) Understand the special problems related to sharps disposal. f) Ensure that waste bags are not overfilled or too heavy g) Ensure that the waste is correctly segregated at all times 18 D:\116103232.doc 22.10 Note: ALL STAFF HAVE A RESPONSIBILITY TO ABIDE BY THE MANAGEMENT OF CLINICAL WASTE POLICY BY USING THE DESIGNATED SHARPS CONTAINERS/BAGS FOR CLINICAL WASTE ONLY. This responsibility includes ensuring that clinical waste is contained safely in the designated collection points thus minimising the risk of exposure to clients, staff or others. On no account are bags or sharps to be placed in corridors or areas accessible to the public prior to their collection. 22.11 These responsibilities must be undertaken with due diligence to ensure that no member of staff or the public are exposed to any substances hazardous to health. 22.12 Waste/Infection Control Lead (where available) a) To be the area “expert” on clinical waste b) To lead by example and promote a climate of continuous improvement c) To identify and correct bad practice d) To promote good practice e) To be supportive and to give feedback appropriately and constructively 22.13 General Responsibilities 22.14 With regard to the infection risks to staff from handling clinical waste, the following are required: a) The bags are handled the least number of times, so far as is reasonably practicable b) Bags/sharps boxes and sealable bins are properly sealed. On no account must orange bags be left unattended at any time whilst in transit on site c) Adequate training is provided for all staff that handle clinical waste d) Where appropriate staff are vaccinated for Hepatitis B e) Adequate facilities are provided for washing hands f) Adequate personal protective equipment and clothing is provided g) Adequate facilities/guidance is provided for washing, changing storage and laundering of contaminated clothing h) Suitable storage units are supplied in which clinical waste is transported and stored; these are to be decontaminated on a weekly basis. 23. STORAGE PRECAUTIONS 23.1 Bags, sharps and sealable bins, when marked, should be taken to a collection point and placed in a suitable dedicated container, i.e. a wheeled trolley or cart for 19 D:\116103232.doc transportation in a dedicated bulk container to a disposal unit. Such containers MUST be kept locked at all times to prevent unauthorised access. 23.2 At all times where manual handling is involved, the necks of the bags should be positioned to allow subsequent movement to be undertaken safely. 23.3 Trolleys and carts used for the movement of clinical waste within the premises should be designed and constructed as 'fit for purpose'. 24. CLEANING OF BULK TRANSPORT ITEMS 24.1 The wheeled bins belong to the waste contractor. The waste contractor will ensure that they are thoroughly cleaned on receipt at the waste facility once emptied of their contents. All empty bins deposited on healthcare sites will be clean or removed and replaced with clean bins at the expense of the contractor. 25. PERSONAL PROTECTIVE EQUIPMENT 25.1 COSHH Regulations require that risks to health be eliminated, prevented or, where this is not reasonably practicable, reduced. Although the use of personal protective equipment should be considered as additional to other control measures, it is likely that even after all reasonably practicable precautions have been taken to reduce the exposure of staff that handle, transfer, transport, treat or dispose of healthcare waste, some personal protective equipment will still be required. 25.2 In such cases, the managers will ensure that these items are provided, used and maintained. They must also make appropriate arrangements for storage and cleaning. 25.3 Under the law, employees must cooperate with employers to ensure that their legal duties are met. 25.4 Emergency situations, such as spillages, will also be addressed in any risk assessments. This might include the need for protective equipment to prevent exposure via routes such as skin contact (for example disposable aprons and gloves) or inhalation (for example respiratory protection and/or face visors). 26. BASIC HYGIENE 26.1 Basic personal hygiene is important in reducing the risk from handling healthcare waste. The manager will ensure that washing facilities are conveniently located for people handling healthcare waste. 27. IMMUNISATION 27.1 Staff handling healthcare waste will be offered appropriate immunisation, including hepatitis B and tetanus. Staff will be informed of the benefits (for example protection against serious illness, protection against spreading illness) and drawbacks (for example reactions to the vaccine) of vaccination. 27.2 Where vaccination has been identified, as a control measure required when working with healthcare waste, manager will advise on access to vaccinations. 20 D:\116103232.doc 28. ACCIDENTS AND INCIDENTS 28.1 All incidents involving spillages damaged packaging, inappropriate segregation or any incident involving sharps need to be reported to the manager or other suitable individual, and be investigated by them. The investigation of these accidents and incidents needs to establish the cause and what action needs be taken to prevent a recurrence. 28.2 The analysis and investigation of incidents involving healthcare waste, whether reportable or not, helps identify causes, trends, the level of compliance with current legislation, the effectiveness of the precautions in place, and problem areas for which satisfactory precautions have yet to be provided. 28.3 The depth of each investigation will vary depending on the nature of the incident. To be worthwhile, however, any investigation needs to consider carefully the underlying causes. Action after an accident will not be effective if it addresses only the superficial and obvious causes and misses more significant issues. 28.4 The active and reactive monitoring of healthcare waste procedures is most effective as part of an overall system of health and safety monitoring. 29. REPORTING OF INJURIES, DISEASES AND DANGEROUS OCCURRENCES REGULATIONS (RIDDOR) 29.1 The Reporting of Injuries, Diseases and Dangerous Occurrences Regulations (RIDDOR) require certain accidents, work-related ill-health and dangerous occurrences (such as an incident that results in, or could have resulted in, the release of a biological agent that could cause severe human disease) to be reported to the appropriate enforcing authority. For most healthcare premises, this is the HSE (http://www.riddor.gov.uk). 29.2 Severe human disease includes diseases caused by hazard group (HG) 3 and 4 agents as well as some (HG) 2 agents (for example Neisseria meningitidis). 29.3 Social security legislation requires an accident book or something similar to be kept and accessible to staff. 30. Mercury 30.1 Departments who use mercury should carry out a risk assessment for dealing with mercury spillages and produce written procedures. A spillage kit including disposable plastic gloves, paper towels, a bulb aspirator for the collection of large drops of mercury, a vapour mask, a suitable receptacle fitted with a seal, and mercuryabsorbent paste (equal parts of calcium hydroxide, flowers of sulphur, and water) needs to be available. In no circumstances should a vacuum cleaner or aspiration unit be used, as this will vent mercury vapour into the atmosphere. 21 D:\116103232.doc 31. 31.1 TRAINING Under Health and Safety at Work legislation, the Management of Health and Safety at Work Regulations and COSHH, staff must receive information on: a) The risks to their health and safety, that is, the details of the substances hazardous to health to which they are likely to be exposed; the significant findings of the risk assessment; b) Any precautions necessary; c) The results of any monitoring carried out; and the collective results of any relevant health surveillance. 31.2 Training on the management of clinical waste, (safe, handling, segregation and the prevention of the spread of infection) should be provided. These sessions are mandatory for all staff as are the annual updates. 31.3 In addition to this, segregation of waste in accordance with this policy will be discussed with new staff members during their local induction. 31.4 Training records 31.5 Training records are kept at in personal files. 32. AUDIT 32.1 The management of clinical waste within the premises will be audited using the tools provided within the Infection Control Nurses Association document ‘Audit Tools for Monitoring Infection Control Standards 2004’. These have been slightly modified to meet standards set since that year. 22 D:\116103232.doc Audit – To be completed by Manager annually. 1 2 3 4 5 6 7 8 9 10 11 12 13 14 17 18 19 20 21 22 23 24 25 26 Management Contractual Arrangements and Documentation There is evidence that the waste contractor is registered with a valid licence (check records) There is an appropriately designated Waste Officer who has undergone training within the last two years (check Job Description and training record) There are completed transfer notes detailing final disposal details for waste collection over the last 12 months Completed consignment notes for hazardous waste detailing final incineration details for waste collection over the last 12 months are available There is annual audit monitoring of the contractor. Check for evidence which includes an audit trail of waste from the site to the incinerator All clinical waste must be transported in UN approved rigid containers There is a dedicated compound for the safe storage of clinical waste, which is under cover from the elements and free from pests and vermin All premises should have a clinical waste storage area away from the public Waste containers are locked and inaccessible to the public The compound is locked and inaccessible to public The compound has appropriate signs in the area Returned containers are clean Containers are in a good state of repair Clinical waste is stored separate to other waste Sharps boxes are correctly sealed Sharps boxes are correctly labelled Sharps boxes are safely stored Biological agents are made safe by autoclaving before leaving the laboratory for final disposal There are no inappropriate items in the household or recycling bins Spill kit & heavy duty gloves or alternative are available There is no storage of inappropriate items in the waste compound The area is clean and tidy (there are cleaning facilities) Clinical waste sacks are labelled and secured before leaving the premises A record is kept of the coded tags used for each premises 23 D:\116103232.doc Yes No N/A Comments 27 28 29 30 31 32 There is no storage of waste in corridors, inside/outside the premises whilst awaiting collection There is a system for transporting the waste through the premises (i.e. which avoids manual handling of waste) Clinical waste is segregated from other waste for transportation All waste containers used for transport are clean All waste containers are in a good state of repair Supplies of mattress bags are available and are used for contaminated mattresses ready for disposal 24 D:\116103232.doc 25 D:\116103232.doc APPENDIX A WASTE SEGREGATION CHART Soft Waste: Orange bag - e.g. gloves, aprons, hand towels, cardboard etc used for healthcare purposes where there is a possibility that they may carry infection Sharps: All sharps in yellow lidded sharps bins - blood or medicinal contamination - except those used to administer cytotoxic/cytostatic drugs Cytotoxic/cytostatic: Sharps and other items contaminated with cytotoxic/cytostatic substances in purple lidded bins Anatomical Waste, Blood Products, Gelling Agents and Larvae: Yellow lidded sealable bins Sensitive Products of Birth: Green lidded sealable bins Domestic Waste - black bag - e.g. flowers, newspapers Household glass and aerosols: Clear plastic bags - e.g. coffee jars and air fresheners 26 D:\116103232.doc APPENDIX B EUROPEAN WASTE CATALOGUE CHAPTERS Chapter Number Production sector/Origin of Waste Chapter 1 Waste from exploration, mining and quarrying, physical and chemical treatment of minerals Chapter 2 Waste from agriculture, horticulture. Aquaculture, forestry, hunting and fishing, food preparation and processing Chapter 3 Waste from wood processing and the production of panels and furniture, pulp, paper and cardboard Chapter 4 Waste from the leather, fur and textile industries Chapter 5 Waste from petroleum refining, natural gas purification and pyrolytic treatment of coal Chapter 6 Waste from inorganic chemical processes Chapter 7 Waste from organic chemical processes Chapter 8 Waste from the manufacture, formulation, supply and use of coatings (paints, varnishes and vitreous enamels), adhesives, sealants and printing inks Chapter 9 Waste from the photographic industry Chapter 10 Waste from thermal processes Chapter 11 Waste from chemical surface treatment and coating of metals and other materials, non-ferrous hydrometallurgy Chapter 12 Waste from shaping and physical and mechanical surface treatment of metals and plastics Chapter 13 Oil and liquid-fuel waste Chapter 14 Waste organic solvents, refrigerants and propellants Chapter 15 Waste packaging, absorbents, wiping cloths, filter materials and protective clothing not otherwise specified Chapter 16 End of life vehicles from different means of transport and vehicle maintenance Chapter 17 Construction and demolition waste Chapter 18 Waste from human or animal healthcare and/or related research Chapter 19 Waste from waste management facilities, off-site waste water treatment plants and the preparation of water intended for human consumption and water for industrial use Chapter 20 Municipal waste (household waste and other similar commercial, industrial and institutional waste (including separately collected fractions) 27 D:\116103232.doc APPENDIX C CYTOTOXIC AND CYTOSTATIC MEDICINES Altretamine capsules Azathioprine tablets Anastrozole tablets Bexarotene capsules Bicalutamide tablets Busulfan tablets Capecitabine tablets Chlorambucil tablets Chloramphenicol drops, capsules minims Colchicine tablets Cyclophosphamide tablets Ciclosporin tablets, liquid Dienestrol cream Diethylstilbestrol tablets Dinoprostone tablets Dutasteride capsules Ergometrine/methylerfometrine tablets, spray Estradiol patches, implants tablets, gel Estramustine capsules Estrogens, conjugated tablets Etoposide capsules Exemestane tablets Finasteride tablets Fludarabine tablets Fluorouracil capsules, cream Flutamide tablets Goserelin implants 28 D:\116103232.doc Hydroxycarbamid Idarubicin capsules Imatinib capsules, tablets Leflunomide tablets Letrozole tablets Lomustine capsules Megestrol tablets Melphalan tablets Mercaptopurine tablets, capsules Methotrexate tablets Mifepristone tablets Mitotane tablets Mycophenolate tablets, capsules Nafarelin nasal spray Procarbazine capsules Progesterone capsules, pessaries, gel Raloxifene tablets Tacrolimus capsules, ointment Tamoxifen tablets Temozolomide capsules Testosterone, patches, implants, capsules Thalidomide capsules Tioguanine tablets, capsules Thiotepa eye drops Torimefene tablets Tretinoin, cream, gel, solution, capsules 29 D:\116103232.doc Valganciclovir tablets Zidovudine capsules, suspension 30 D:\116103232.doc APPENDIX D COMMUNITY CARE Community care can take many forms and occurs in various environments. It includes healthcare workers (including emergency care practitioners (ECPs)) who provide care and support to: a) patients in their own homes; b) residents of care homes (without nursing care); c) householders who are self-medicating. As a community practitioner, the following types of waste will be produced: a) infectious; b) sharps; c) medicinal; d) anatomical (for example placentas); e) domestic. Colour-coding The colour of the waste receptacle will depend on how the waste should be treated and disposed of: Orange – sacks should be used for waste that can be treated to render it safe. In practice, the vast majority of “soft” infectious waste such as dressings, bandages and some plastic single-use instruments can be treated. Yellow – yellow-lidded sharps receptacles and sealed units as appropriate should contain waste that requires disposal by incineration only. A relatively small amount of waste produced in the community will be disposed of in yellow containers; examples of waste materials include anatomical waste (such as placentas) and sharps. Yellow/purple –purple-lidded sharps receptacles should be used for waste that is contaminated with cytotoxic and cytostatic medicinal products. In the community setting this will include sharps used for the administration of chemotherapy, antiviral and/ or hormonal drugs. Black – used for mixed domestic waste – it should never be used for recognisable healthcare waste. Which type of packaging and what colour should you use? Packaging The type of packaging used will vary on the type of waste produced: if the waste is liquid or contains free liquids (for example a partially discharged syringe), it should only be placed in a package designed to take liquids, such as a plastic drum; if the waste is sharp it should 31 D:\116103232.doc only be placed in a sharps receptacle (see below); all other waste may be packaged in flexible sacks (infectious waste bags). It is not always practical for healthcare workers to carry lots of different types of packaging with them. Therefore, healthcare workers must choose the most appropriate packages to meet their needs. Colour Orange sacks The vast majority of “bagged” infectious waste produced in the community will be placed in the orange waste stream. Therefore, the use of orange sacks in the community is recommended. Small rigid leak-proof yellow receptacles Where anatomical or other waste that requires incineration is being generated, it will be appropriate for healthcare workers to carry yellow packaging. As most “incineration only” waste is either anatomical or sharps and/or contains lots of free liquid, the use of small rigid yellow boxes is recommended. These should have purple lids if the waste is contaminated with cytotoxic/cytostatic medicines (see below for appropriate colour-coding of sharps). DISPOSAL OF PHARMACEUTICAL WASTE IN DOMICILLARY SETTINGS Patients or their relatives should be encouraged to return unwanted medication to a community Pharmacy (or Dispensing Practice) for safe disposal. The PCT contracts with a waste carrier to make regular collections of pharmaceutical waste from these settings and the pharmacy contract and Dispensing Services Quality Scheme include mechanisms for checking that such waste is correctly handled. The waste service provides separate bins for the disposal of hazardous waste and it is responsibility of the Pharmacy or dispensing practice to ensure that appropriate segregation of hazardous waste occurs. Patient returned medication should not be accepted by GP practices or other community based clinics. It should all be referred to community pharmacies (or dispensing practices) Community Pharmacies cannot accept sharps waste (or other as part of an Enhanced Service Needle Exchange Programme). When a patient is prescribed injectable medication or administration in their own home, the GP should prescribe a sharps bin on an FP10 prescription. Sharps bins filled to the recommended level should be sealed and returned to the GP practice for safe disposal. It is the responsibility of the General Practices to ensure safe disposal of sharps waste returned to their premises. Pharmaceutical waste generated by a clinic GP practice or other healthcare organisation such as home care with nursing must be disposed by that organisation following national policy and cannot be accepted by community pharmacies. 32 D:\116103232.doc Infectious waste Waste classified as infectious waste due to its known or potential risk of infection should be classified as hazardous infectious waste and should be packaged appropriately and sent for suitable treatment and disposal. Non-infectious dressing assessment Where assessment has identified that the dressing is not infectious, the following should be considered (noting that the type of dressings that are produced in the community by a healthcare worker can vary greatly): 1. any recognisable item of non-infectious healthcare waste cannot legally be disposed of in the black-bag waste stream and should therefore be disposed of in the clinical waste stream; 2. however, mixed domestic waste does contain small amounts of plasters, small dressings and incontinence products. Where the healthcare worker produces the same or similar items, these – with the following considerations – can be placed in the domestic refuse (with the householder’s permission). The following should be considered: the size of the dressing – small dressings no larger than a dressing pad (that is, 130 mm × 220 mm) can be disposed of as domestic refuse; the type of dressing – specialised antimicrobial types of dressing should be disposed of as offensive/hygiene or medicinal waste as appropriate; the quantity produced – where a number of small dressings are produced regularly over a period of time, it may be appropriate to dispose of these as offensive/hygiene waste. If, however, the amount produced is relatively small and consistent with that likely to be found in the household waste stream, it may be discarded in the domestic refuse; packaging – where such waste is placed in the domestic refuse, the waste should be wrapped in a plastic sack. The wrapping should not be yellow or orange, as the waste is not deemed to be infectious – thin opaque plastic sacks such as sandwich bags and bin liners are appropriate. Miscellaneous infectious waste Purple-lidded sharps receptacles. Community practitioners are likely to administer a wide variety of medicinal products by injection. Some of these will be classified as cytotoxic and cytostatic; therefore, the associated sharps and liquid residues of the medicinal products should be placed in an appropriate yellow and purple leak-proof sharps box. Yellow-lidded sharps receptacles. If you have determined that none of the products used for injection is classified as cytotoxic or cytostatic, it is appropriate to use a yellow-lidded sharps box. For reasons of practicality, community nurses may seek to use a single sharps receptacle. In this instance, it would need to be a yellow/purple leak proof sharps receptacle. 33 D:\116103232.doc Notes: Sharps should never be discharged to allow disposal into a certain type of box. Sharps boxes should be collected when three-quarters full and must never exceed the permissible marked mass. If the sharps box is seldom used, it should be collected after a maximum of three months regardless of the filled capacity. Biological substances Category B (formally diagnostic specimens) are not considered to be waste unless discarded at laboratory facilities. Diagnostic specimens should be placed in specimen packs for transport (packaging instruction P650 and labelled UN3373). The packaging used to transport the specimens does not require UN approval providing the containers meet the requirements of P650. Self-medicating patients and sharps disposal Where the householder is a self-medicating patient who uses injectables (for example a person with diabetes) with no healthcare worker involved in the administration, the GP or healthcare worker should prescribe the householder the appropriate container (for example a sharps box) and advise them of local disposal options. The householder should be trained in how to use the sharps box before it has been prescribed, to ensure that they understand its use and ensure it is correctly sealed and labelled. Once the sharps bin is three-quarters full, it should be sealed by the householder and taken back to the GP surgery or to the local pharmacy for disposal. Note: It is no longer acceptable to advise self-medicating patients to dispose of their lancets into the households black-bag waste stream. Patients with MRSA Where a patient in the community has been diagnosed with MRSA and is being cared for by a healthcare worker, the healthcare waste generated is not necessarily infectious. In assessing the risk of infection from waste produced by a patient with MRSA, the following should be considered: Is the patient colonised with MRSA but not receiving specific treatment for MRSA? If the answer is “yes”, the MRSA status of the patient does not effect the assessment of the waste. The healthcare worker should refer to the wound and dressing assessment given in above. Is the patient infected with MRSA and receiving treatment, and is the infection present in the waste generated? If the answer is “yes”, the waste produced should be classified as infectious waste. 34 D:\116103232.doc Disposable instruments Disposable instruments are now commonly being used in the community by a number of healthcare professionals. Disposable instruments can take the form of either plastic or metal instruments. Contaminated plastic disposable instruments – where there is no risk of sharps – can be safely disposed of as infectious waste and put into the orange-bag waste stream. Metal disposable instruments – where there is no risk of sharps and they are deemed to be infectious – should be put into a rigid yellow container marked “for incineration only”. Note: Disposable instruments cannot legally be disposed of in the domestic refuse. Stoma/catheter bags If a healthcare worker is involved in the care of a stoma site, the waste from a stoma patient can be disposed of in the black-bag waste stream. If used in bulk, this becomes offensive/hygiene waste for disposal in black/yellow-striped bags for deep landfill. However, if the person develops any type of gastrointestinal infection or the site becomes infected, the bag must be disposed of as infectious waste into the orange-bag waste stream. If the householder is self-medicating with no healthcare worker involved, they are able to dispose of their own waste in to the black-bag waste stream. Wound vacuum drains These should be treated as infectious waste and disposed of in the orange-bag waste stream. They should never be placed in the domestic refuse. Maggots All maggots used for wound management should be secured in an airtight rigid yellow container and marked as UN 3291. Transporting the waste Where a healthcare worker in the community generates waste, the healthcare worker is responsible for ensuring that the waste is managed correctly; this is part of their duty-of-care. Where the waste is generated in other premises, such as in care homes and private households, the healthcare worker must ensure that arrangements are in place to ensure that the waste is packaged and labelled correctly and transported for appropriate treatment and disposal. Local options may vary, but in general the healthcare worker has two options. Option 1 The healthcare worker producing the waste can transport the infectious waste from the home environment back to base where waste collection and disposal arrangements are in place. Where healthcare workers are transporting waste in their own vehicles, they should 35 D:\116103232.doc ensure that they are transporting the waste in secure rigid packaging, for example boxes or drums (following packaging instruction P621). They should also have received appropriate training either in-house or contracted out, which addresses the transporting of waste safely. Option 2 The healthcare worker producing the waste can leave it in the home for collection by an appropriate organisation, either a waste contractor or the local authority or healthcare provider. The healthcare worker has responsibility for the waste while it is being stored awaiting collection, and for arranging that collection. While awaiting collection from the householder’s home, the waste should be stored in a suitable place to which children; pets, pests etc do not have access. It is not appropriate to leave the waste unsupervised on the pavement awaiting collection. Waste should be packaged and labelled appropriately and adequate instruction should be given. The party collecting the waste should be provided with the information required under dutyof-care requirements. A completed consignment note should accompany the movement of the waste, as infectious waste is classified as hazardous waste. Note: A consignment note is not required for the movement of hazardous waste from domestic premises. 36 D:\116103232.doc POLICY APPROVED BY: JOB TITLE PRINTED NAME SIGNATURE DATE Clinical Policy Review Group Signed by Chair on Behalf of the Group POLICY AUTHORISED BY PRINTED NAME AUTHORISING MANAGER (First) Ian Brennan Associate Director of Community Health Services AUTHORISING MANAGER (Second) Brian Goodrum, Associate Director of Mental Health Services SIGNATURE AUTHORISING MANAGER (Third)* DATE APPLICABLE REVIEW DATE PERSON RESPONSIBLE FOR REVIEW * if applicable 37 D:\116103232.doc DATE