Sunflower atypical transcription factors and miRNAs playing
advertisement

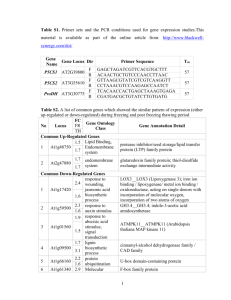
Sunflower atypical transcription factors and miRNAs playing a key role in responses to abiotic stresses Agustín L. Arce*, Jorge I. Giacomelli*, Karina F. Ribichich, Raquel L. Chan § Instituto de Agrobiotecnología del Litoral, Universidad Nacional del Litoral, CONICET, CC 242 Ciudad Universitaria, 3000, Santa Fe, Argentina. *These authors equally contributed to this manuscript § Corresponding author (rchan@fbcb.unl.edu.ar) ABSTRACT Background to the research and aims Along the history of Plant Sciences, plant models have been chosen for most studies and these plant models have been selected based on their genome size, transformation feasibility, life cycle length and other features. By these selection parameters, sunflower appeared as inadequate for most molecular approaches (large and unknown genome, difficult to transform, etc.) and hence, molecular research in this species remained largely delayed compared to that of Arabidopsis, rice and other model plants. Due to the great economic importance of this species in Argentina and in spite of the multiple difficulties that molecular studies in sunflower implicate; since 1993 our research group has been devoted to the investigation of the molecular mechanisms triggered by this plant to regulate its gene expression. Aiming to understand how sunflower deals with abiotic stress factors, we started our investigation with key players of the response to such stresses, the transcription factors. Overview of the methodology used A series of Molecular Biology techniques were used to performed this study. These techniques and strategies include databases analysis, phylogenetic trees construction, screening of genomic DNA libraries, isolation of cDNA clones, expression studies using Norhtern blots, western blots and qRT-PCR, functional analyses using plant transformation, both stable and transient, confocal microscopy and microarrays. Key results Transcription factors are proteins able to recognize and bind specific DNA sequences present in the regulatory regions of their target genes. Upon binding, entire signalization cascades are induced or repressed and the plant can adapt itself, at least temporarily, to the adverse conditions to which it is subjected. Plant transcription factors (TFs) were classified in families and subfamilies according to the structure of the binding domain as well as to other structural features. Members of the homeodomain-leucine zipper (HDZip) and WRKY families have been functionally characterized in our laboratory. Phylogenetic trees constructed with sequences from many species indicated, besides the conserved members, the existence of divergent transcription factors in the Asteraceae family. The stated hypothesis is that these proteins may be playing differential and specific functions in these plants. Examples of proteins without orthologues in model plants are the HD-Zips HaHB4 and HaHB11 and members of the novel WRKY clade, HaWRKY75 and HaWRKY76. In this sense, we were able to demonstrate that the HD-Zip HaHB4, not presenting ortologues in the model plants, confers tolerance to drought, salinity and herbivory attack via the repression of ethylene perception. HaWRKY6 participates in the abiotic stress response and is post-transcriptionally regulated by a miRNA. This last regulation mechanism does not take place in Arabidopsis for HaWRKY6 putative homologues. Conclusions The most amazing results obtained during these studies and others currently carried on are related to the divergence in structure and function of TFs and miRNAs found in sunflower, apparently conserved in some cases in other Asteraceae species but not in model plants. The release of the genomic sequence together with the advance in transformation techniques will certainly help to better understand how sunflower evolved to be adapted to abiotic stress factors and which novel regulating molecules are playing key roles in such adaptation. Contribution The results described here contributed to a better understanding of the regulation of gene expression in sunflower. Additionally, these studies highlighted the importance of the research in non model plants. KEY WORDS: transcription factors – WRKY - HD-Zip – miRNAs OVERVIEW AND SCOPE OF THE PRESENTATION Most of the molecular studies, about gene expression and regulation, appearing in the scientific literature and specially those published in high impact journals have been performed in model plants. The genome of the plant model Arabidopsis was the first to be sequenced and released by 2000. After that, several plant genomes including monocots like rice and maize, barrel clover (more recently) and others became available in public databases. Model plants present other advantages for their study at the molecular level: the availability of established transformation protocols, mutant and ESTs libraries, commercial microarrays, etc. In spite of their agronomic importance, working with sunflower and other Asteraceae members exhibits enormous difficulties; there are no easy transformation protocols, nor available genome neither commercial microarrays. These considerations lead to the state the question: why sunflower? Possibly, the first intuitive response implies the economic importance, especially in our country, Argentina. However, this would not be the more accurate response but one considering the interest in such beautiful and interesting species that after a simple or deep sight is so different from Arabidopsis, rice or barrel clover. As in other species, the regulation of gene expression in sunflower involves major actors: transcription factors (TFs), genomic regulatory regions and more recently, small RNAs including miRNAs. External factors, both from biological or abiotic origins, affect the levels of specific proteins, by transcriptional and or post transcriptional regulations. TFs are proteins able to recognize and bind specific DNA sequences (cis-acting elements) present in the regulatory regions of their target genes, and modulate their transcription. MiRNAs are small RNA molecules of ~21 nucleotides which direct the cleavage or translational inhibition of specific mRNAs. They originated from primary longer RNA transcripts that include a self-complementary fold back, from which the mature mirRNAs are excised. It is accepted that the regulation of gene expression mostly follows conserved mechanisms between plant species. In this sense, it is expected that homologous genes from different plants present similar expression patterns and this similarity defines their orthology. However, species like sunflower exhibit differential characteristics determined by unique genes that do not have orthologs in model plants or alternatively by unique regulation mechanisms. These genes are playing regulatory roles unique to this species and seem to be conserved only in other Asteraceae. Along this manuscript, some examples of such divergence in the regulatory mechanisms will be presented and discussed. We propose the divergent genes that participate in this regulation of gene expression trigger a more sophisticated biological system able to respond to different external adverse factors. OUTLINE OF RECENT MAJOR DISCOVERIES Transcription factors Plants, as sessile organisms unable to move when the external conditions are adverse, evolved triggering different and complex defence mechanisms which allow them to survive in such conditions for variable periods of time. The ability of a given species to survive it is intimately related to a series of molecular responses involving activation and repression of certain genes. The response regulatory network turned out to be very complex. Stress tolerance seems to be controlled mostly at the transcriptional level, depending on TFs activity. TFs have a modular structure and exhibit at least two types of domains: a DNA binding domain and a proteinprotein interaction domain which mediates, directly or indirectly, the activation or repression of transcription (Brivanlou and Darnell, 2002). Approximately 2000 plant TFs were in silico identified in plants and classified in families and subfamilies according to the similarity in the binding domain, their gene structure, their function and other structural features. Among these identified TFs, only a little percentage has been functionally characterized, even in model plants. Among these groups, the WRKY and the HD-Zip members were related to the abiotic and biotic stress response. Some sunflower TFs belonging to these families have been characterized and are shortly described below. The HD-Zip family The HD-Zip family is composed of proteins bearing a homeodomain (HD) associated to a leucine zipper (LZ), association unique to plants whereas the separated domains are present in TFs of other kingdoms. This family is divided in four groups according to the conservation of the HD-Zip domain, the presence of additional conserved domains, the gene structure and the pathways in which these proteins participate. Members from the subfamily I have been characterized as active participants in the adaptive response to several abiotic stresses and the expression of most of them is regulated by drought, salt and ABA in different tissues/organs (Henriksson et al., 2005; Ariel et al., 2007). When some of these proteins were overexpressed, they provoked phenotypic changes associated with abiotic stress tolerance. The sunflower HaHB4 was thought to be the Arabidopsis AtHB7/12 ortholog. However, when these genes were overexpressed in Arabidopsis plants, the new phenotypes were clearly different (Dezar et al., 2005; Manavella et al., 2006; Cabello et al., 2007; Manavella et al., 2008). A new phylogenetic tree of 178 HD-Zip I proteins together with the sequence conservation presented outside the HD-Zip domains allowed the distinction of six groups of proteins (Arce et al., 2011). A motif-discovery approach enabled the recognition of an activation domain in the C-terminal regions s and some putative regulatory mechanisms acting in the N-terminal regions and C-terminal regions s involving sumoylation and phosphorylation. Notably, some sunflower members belonging to one of these groups exhibit atypical C- and N- termini. Sunflower HaHB4 and HaHBX genes (Figure 1) presented the ability to confer several agronomic traits in response to environmental stressed. Notably, no orthologues of these genes were detected in model plant species. Figure 1. HAHB4 AND HAHBX from sunflower represent divergent members of group I of HD-Zip I proteins The phylogeny of Arabidopsis thaliana HD-Zip I proteins illustrates the presence of different groups (in roman numbers) of structurally and functionally related proteins within the subfamily I of HD-Zip transcription factors. These groups arose from a study of 178 proteins from several species, including monocots and dicots, and have been defined based on phylogenetic relations and shared motifs outside the HD-Zip domain. This allowed the characterization of a region responsible for transcriptional activation in yeast, and the identification of putative phosphorylation and sumoylation sites. Recently, another posttranslational modification, ubiquitination, has been discovered to play an important role in the regulation of ATHB6 and possibly other HD-Zip proteins. HAHB4 AND HAHBX are two sunflower proteins phylogenetically belonging to group I which are structurally divergent. They lack the conserved motifs present in other members of the group, as exemplified in the figure by Arabidopsis ATHB12 and ATHB7. They also lack putative sumoylation sites (S). Not surprisingly, the phenotype of Arabidopsis plants which overexpress these proteins is substantially different from plants which overexpress ATHB12 and ATHB7. In contrast, the phenotype of Arabidopsis plants overexpressing Arabidopsis ATHB13 or sunflower HAHB1, both typical members of group V, is highly similar. The WRKY family The WRKY family includes proteins mainly characterised by the presence of a 60 amino acid conserved region containing the four WRKY amino acids and a zinc-finger-like motif, which together form the WRKY domain. These proteins are unique to plants and have been classified in Arabidopsis on the basis of both the number of WRKY domains and the pattern of their zinc-finger-like motif into three main groups (I, II and III), the second of which is divided in five subgroups, IIa, IIb, IIc, IId and IIe (Eulgem and Somssich, 2000; Rushton et al., 2010). The huge quantity of expressed sequence tags (ESTs) from Helianthus spp. available in public databases allowed us to identify 97 sunflower WRKY members using bioinformatics, and to assign them putative orthologues from model plants (Giacomelli et al., 2010). Phylogenetic trees constructed with WRKY domains resolved seven groups as in Arabidopsis and rice and additionally, a novel clade with traits apparently specific to Asteraceae. This indicates that the WRKY family has undergone a particular diversification, which could be the source of specific new functions in these species. The members of this new clade includes proteins that are divergent from the group IId presenting common motifs like the C motif, a zinc cluster and a HARF motif but in addition, they present a conserved N-terminal domain, a serine –rich region, a PPLP motif and a WKKYGEK domain instead of the canonical WRKYGQK domain. These motifs and domains are unique to members of this new clade. A comparison between groups IId and WKKY are presented in Figure 2. Common structures would possibly determine conserved functions while exclusive stretches could be the basis of neo-functionality. The WKKY motif is probably the most remarkable feature of this novel group. Although the K to R is a conservative substitution, it could disturb the DNA-protein interaction: for example KR to RK substitutions completely abolished the interaction between AtWRKY11 and its target W-box (Ciolkowski et al., 2008). Further studies must be performed to corroborate the hypothesis of neo-functionalization; some of them are currently carried out or will be informed in the near future. AIQEAASSGLxSxE[….]LLS PxxP(x)6PPL PPDEYSWRKYGQK[…]Cx(4-5)Cx(22-23)Hx1H N C H P Z WRKYGQK N C H P Z WKKYGEK Figure 2. Putative conserved primary structural features of Arabidopsis WRKY IId proteins (above) and Helianthus/Lactuca WKKY (WKKYGEK) motif encoding-proteins (below). Sequences of Arabidopsis best characterized WRKY IId proteins were used for the above picture. ESTs from Helianthus spp. and Lactuca spp. were assembled in EST-clusters, were translated thereafter and used for the below picture. Geometric designs and labels indicate features identified by using MEME. Boxes (blank) identify conserved domains between WRKY IId and WKKY proteins: C, the calmodulin-binding motif; H, the HARF motif; Z, the zinc cluster. Boxes with rounded corners identify WRKY IId motifs (dark grey) and WKKY motifs (light grey): N, the Nterminal consensus sequence; P, proline-rich motif; W(R/K)KYG(Q/E)K, the W(R/K)KY domain, which includes potential zinc ligands (C2-H2). The S-Rich Region indicates the serine-rich stretch, a variable region with several serines at both ends, only found in WKKY proteins. The miRNAs Although transcription is the most important point of regulation, post-transcriptional silencing via different mechanisms also takes place in plants. The majority of the Arabidopsis miRNAs is evolutionarily young and not present in other plant species, suggesting that birth and loss of these small RNAs is a frequent process (Fahlgren et al., 2007). It is not clear whether these recently evolved miRNAs have a relevant biological contribution. Sunflower miRNAs and small RNAs have not been described yet. However, it is assumed that like in other species, these molecules exist and play crucial roles in gene regulation. A bioinformatic search in public databases allowed the identification of divergent genes. For example hanmiR396 exhibits a mismatch with its homolog in Arabidopsis indicating a possible target evolution for these molecules. This mismatch was not detected in lettuce or other Asteraceae species (Figure 3) indicating a divergence exclusive of sunflower. Moreover some miRNAs detected in sunflower and Asteraceae species present hits only with members of the moss Physcomitrella patens (http://www3a.biotec.or.th/micropc/) but not in vascular plants. Figure 3. Divergent sunflower and other Asteraceae miRNAs a- Sequence comparison between miR396 from sunflower, lettuce and Arabidopsis; b) predicted structure for han-miR396; c) sequence comparison between members of Helianthus generous and Physcomitrella patens (hex = Helianthus exilis, ppt = Physcomitrella patens, htu = Helianthus tuberosus, han = Helianthus annuus; d) predicted structure of hex-miR1040 SIGNIFICANT PERCEIVED GAPS IN RESEARCH, CURRENT DEBATES, AND PERSPECTIVES FOR FUTURE DIRECTIONS OF RESEARCH In conclusion, the Asteraceae family and particularly the sunflower, less studied at the molecular level and presenting largely unknown genome sequences, exhibit unique genes when compared with model plants genomes. The identified genes encode transcription factors and miRNAs, both playing key roles in the regulation of gene expression. Interestingly, conserved miRNAs generally target transcription factors families making them master regulators of processes such as plant development, hormone signaling and stress response (Axtell, 2008). Further studies and the knowledge of the sunflower genome to be released in the near future will help to elucidate in which processes these molecules are involved. Our group will continue with functional genomic approaches in order to contribute in this fascinating area. REFERENCES CITED IN THE TEXT Arce, A.L., Raineri, J., Capella, M., Cabello, J.V., and R.L. Chan. (2011) Uncharacterized conserved motifs outside the HD-Zip domain in HD-Zip subfamily I transcription factors; a potential source of functional diversity. BMC Plant Biol. 11: 42-42. Ariel, F.D., Manavella, P.A., Dezar, C.A., and R.L. Chan. 2007. The true story of the HD-Zip family. Trends Plant Sci. 12:419-426. Axtell, M.J. 2008. Evolution of microRNAs and their targets: are all microRNAs biologically relevant? Biochim. Biophys. Acta 1779: 725-734. Brivanlou, A.H., and J.E. Darnell. 2002. Signal transduction and the control of gene expression. Science (New York, N.Y.), 295:813-818. Cabello, J.V., Dezar, C.A., Manavella, P.A., Chan, R.L. 2007. The intron of the Arabidopsis thaliana COX5c gene is able to improve the drought tolerance conferred by the sunflower Hahb-4 transcription factor. Planta 226:1143-1154. Ciolkowski, I., Wanke, D., Birkenbihl, V., and L.E. Somssich. 2008. Studies on DNA-binding selectivity of WRKY transcription factors lend structural clues into WRKY-domain function, Plant Mol. Biol. 68:81-92. Dezar, C.A., Gago, G.M., González, D.H., and R.L. Chan. Hahb-4, a sunflower homeobox-leucine zipper gene, confers drought tolerance to Arabidopsis thaliana plants. Transgenic Res. 14:429-440. Eulgem, T., Rushton, P.J., Robatzek, S., and I.E. Somssich, The WRKY superfamily of plant transcription factors, Trends Plant Sci., 5 (2000) 199-206. Eulgem, T., and I.E. Somssich. 2007. Networks of WRKY transcription factors in defense signaling, Curr. Opin. Plant Biol. 10:366-371. Fahlgren, N., Howell, M.D., Kasschau, K.D., Chapman, E.J., Sullivan, C.M., Cumbie, J.S., Givan, S.A., Law, T.F., Grant, S.R., Dangl, J.L., and J.C. Carrington. 2007. High-throughput sequencing of Arabidopsis microRNAs: evidence for frequent birth and death of MIRNA genes. PloS One, 2, e219-e219. Giacomelli, J.I., Ribichich, K.F., Dezar, C.A. and R.L. Chan. 2010. Expression analyses indicate the involvement of sunflower WRKY transcription factors in stress responses, and phylogenetic reconstructions reveal the existence of a novel clade in the Asteraceae. Plant Sci. 178: 398-410. Henriksson, E., Olsson, A.S., Johannesson, H., Johansson, H., Hanson, J., Engstrom, P., and E. Soderman. 2005. Homeodomain leucine zipper class I genes in Arabidopsis. Expression patterns and phylogenetic relationships. Plant Physiol. 139:509-518. Manavella, P.A., Arce, A.L., Dezar, C.A., Bitton, F., Renou, J.P., Crespi, M., and R. L. Chan. 2006. Cross-talk between ethylene and drought signaling pathways is mediated by the sunflower Hahb-4 transcription factor. Plant J 48:125-137. Manavella, P.A., Dezar, C.A., Bonaventure, G., Baldwin, I.T., and R.L. Chan. 2008. HAHB4, a sunflower HD-Zip protein, integrates signals from the jasmonic acid and ethylene pathways during wounding and biotic stress responses. Plant J. 56:376-388. Rushton, P. J, Somssich, I.E., Ringler, P., and Qingxi J Shen. 2010. WRKY transcription factors. Trends in Plant Sci. 15:247-58.