Metal nanoparticle formation in oil media using di(2
advertisement

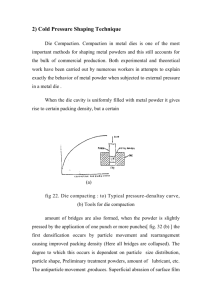
Formation of nano Ni particle in oil medium using di(2-methylhexyl) phosphoric acid (HDEHP) A. Shokuhi Rad Department of Engineering, Chemical Engineering group, , Islamic Azad University, ghaemshahr Branch, GHAEMSHAHR, IRAN shokohiradali@yahoo.com Abstract HDEHP is regularly employed in liquor membrane extraction as metal ion extracting and has been exposed to stabilize nickel (Ni) nanoparticles in oil medium of size 3_13 nm. The particle are shaped by reduce of the metal salt of HDEHP that are oil soluble and revealed by SANS to shape small upturned micelles. The extracting is moreover successful at stabilizing particles of parallel size in an oil phase while the metal ion (e.g., Ni (NO3)2) is reduced in a simultaneous aqueous phase. Keywords: Nanoparticles; Nickel; HDEHP 1. Introduction In current years there has been noteworthy attention in pattern of metal nanoparticles in oil media through reduction of metal salts in the attendance of a stabilizing mediator (Cushing et al.2005). a number of the initial schoolwork concerned water-inoil (w/o) microemulsions somewhere the metal ion salts (e.g., Pt) and reducing agent were detached as aqueous solutions in divide microemulsions that were then mixed (Clint et al. 1993). The quick communication among droplet (Fletcher et al. 1983) consequences in fast mixing of the reactants facilitate nucleation and enlargement of particles within the microemulsions droplets which served to in cooperation stabilize the particles and to several extent limit their enlargement. Nevertheless, the generally solubility of the metal salts employed within the microemulsion imperfect the yield of particles that could be shaped by this method. Pileni has developed Anionic surfactants to fabricate copper and silver nanoparticles using the di-chained surfactant bis(2-ethylhexyl) sodium sulfosuccinate (AOT) where the sodium is replaced, or somewhat replaced, by the pertinent metal ion and reducing agent (e.g., hydrazine) added. The water content (w = [H2O]/[AOT]) of the microemulsion here supply supplementary manage for couture the dimension of spheroid particles (Lisiecki et al.1993) and formation of rod-shaped particles. Other parameters (salt content (Filankembo et al. 2000), anion identity (Filankembo et al. 2003), and hydrazine concentration (Salzemann et al. 2004) were also shown to influence growth of long rod shaped particles and nanocrystal morphology. This revolutionary work produced small ~2-10 nm particles that could be desiccated and re-dispersed. Di (2-ethylhexyl) phosphoric acid is an oil-soluble metal ion extractant that has establish function in liquid membrane processes for the discriminating extraction of metal ions from water into an oil phase (Danesi 1984 and Yu et al. 1998) . Since HDEHP has a similar structure to AOT, metal salts of this weak acid may also be expected to form reversed micelles in oil. 2. Materials and methods 2.1. Preparation of Nickel DEHP salts The common technique used to practice Nickel DEHP metal salts complicated neutralization of HDEHP with the precipitated metal hydroxide in a two phase extraction process: (1) extraction M(OH)n(s,aq) +nHDEHP(org)→M(DEHP)n(org) +nH2O. Di(2-methylhexyl) phosphoric acid (97%, Aldrich) was at first disinfected along with the technique of Partridge et al. (1969). An unadulterated form of the nickel salt of DEHP is formed in this method and a sample was detached for these revise. Ni(DEHP)2 was organized subsequent the methods described previously for preparation of di- and tri-valent metal salts (Steytler et al. 1996). Nevertheless, since the Ni(DEHP) salt is reduced in an oil phase via hydrazine , it can alternatively be newly prepared in situ in relation to the exceeding design and the oil phase alienated following washing by water. (2) aqueous phase M(NO3)n +nNaOH→M(OH)n + nNaNO3, 2.2. Training of nanoparticles from (DEHP) salts In a distinctive training overload aqueous hydrazine (94%, 25 μl) was further to 5 ml of a 0.04 M solution of the Ni(DEHP)2 salt in cyclohexane at room temperature. After that the mixture was shaken, to allowing phase separation. Following separation the colored oil phase containing the particles was detached. 3. Results and discussion 3.1. Structure of Ni(DEHP)2 salts in oil Fig. 1 shows SANS from Ni(DEHP)2 in cyclohexane which characteristic of small aggregates . DEHP salts of di- and trivalent metals in cyclohexane were beforehand investigated using SANS (Partridge et al. 1969). Ni(DEHP)2 reverse micelles in deuterated cyclohexane with a radius of 1nm and a length of 20 nm which show a pole structure. Dependent on concentration of Ni(DEHP)2 be bequeathed view to demonstrate changes in micelle structure. While in the waterless state, underneath a concentration of about 0.035 g cm−3 the Nickel salt subsist either as a monomer or small clusters of limited aggregation number. More than this rank the overturn micelles shaped is pole shaped. The addition of water to this system causes the arrangement of spherical micelles at the higher surfactant levels. For the divalent metal salts, the uptake of water in the system has been established to be extremely small causing just hydrated reverse micelles to be formed. 3.2. Formation by reduction of (DEHP) salts Fig. 2 shows size-distribution of particles obtained by reduction of Ni(DEHP)2 in cyclohexane. On adding of hydrazine, the clear apparent solution of the Ni(DEHP)2 salt slowly darkened to an strong profound brown color trait of the attendance of colloidal Nickel particles. This was go together with evolution of gas according to: Ni(DEHP)2(org) +N2H2(aq) →Ni(org) +2HDEHP(org) +N2(g) Following being separated from the aqueous phase, the resultant metal nanoparticles appeared to be stable over a period of 3 days or more in the oil phase. Conclution By reduction Nickel metal salt of HDEHP inside an oil phase by adding of hydrazine, consequences in configuration of stable Ni nanoparticles. Reversed micelles shaped through these forerunner organometal salts show low aggregation numbers (<5) representative that particle configuration is not forced by the reversed micelle size. Particles of analogous dimension can also be formed by a two phase extraction procedure wherein the more willingly obtainable inorganic water soluble salt is reduced in aqueous solution in make contact with an HDEHP-containing oil phase. Now particle configuration in the aqueous phase is quickly followed through extraction into the oil phase and stabilization by HDEHP. The variation in reducing agent (hydrazine vs sodium borohydride) engaged and the stabilizing agent (HDEHP vs DEHP−) can clarify why smaller particles are given by this technique comparative to those obtained previously (Steytler et al., 1996) References: Clint, J. H.; Collins, I. R.; Williams, J. A.; Robinson, B. H.; Towey, T. F.; Cajean, P.; Khan-Lodhi, A. "Synthesis and Characterization of Colloidal Metal and Semiconductor Particles Prepared in Microemulsions" Chem. Soc. (1993), 95, 219. Cushing, B.L; Kolesnichenko, V.L; O’Connor, C.J. "Recent Advances in the. LiquidPhase Syntheses of Inorganic Nanoparticles"Chem. Rev., (2004), 104(9), 3893. Danesi,P.R."Separation of metal species by supported liquid membranes" Sep. Sci. Technol. (1984) 19, 857. Filankembo, A.; Giorgio, S.; Lisiecki, I.; Pileni, M.P. "Is the anion the major parameter in the shape control of nanocrystal?" J. Phys. Chem. B (2003) 107, 7492. Filankembo, A; Pileni, M. P. " Shape control of copper nanocrystals"Applied surface science (2000) 164(4), 260 Fletcher, P.D.I.; Robinson; B.H. "Ligand exchange kinetics in ternary and quaternary surfactant systems" J. Chem. Soc. (1983) 79, 1959. Lisiecki, I.;Pileni; M.P. "Synthesis of copper metallic clusters by using reverse micelles as microreactors" J. Am. Chem. Soc. (1993) 115, 3887. Partridge, J.A.; Jensen, R.C."Purification of di-(2-ethylhexyl)phosphoric acid by precipitation of copper(II) di-(2-ethylhexyl)phosphate"Journal of Inorganic and nuclear chem. (1969) 31(8), 2587 Salzemann, C.; Lisiecki, L.; Urban, J.; Pileni, M.P. "Anisotropic copper nanocrystals synthesized in a supersaturated medium : nanocrystal Growth" Langmuir (2004) 20, 11772. Steytler, D.C.; Jenta, T.R.; Robinson, B.H.; Eastoe, J.; Heenan, R.K."Structure of Reversed Micelles Formed by Metal Salts of Bis(ethylhexyl) Phosphoric Acid " Langmuir (1996) 12 1483. Yu, Z.J.; Ibrahim, T.H.; Neuman, R.D."Aggregation behaviour of cobalt(Il), ... phosphate complexes in n-heptane" Solvent Extr. Ion Exch. 1998, 16 (6), 1437 Figures: Fig. 1. SANS from Ni(DEHP)2 in cyclohexane at 3% w/v; (a) dry, (b) following shaking with a surplus aqueous phase. Fig.2. Size-distribution of particles obtained by reduction of Ni(DEHP)2 in cyclohexane( the results obtained from TEM image of obtained Ni particles)