Sludge Separation Experiment
advertisement

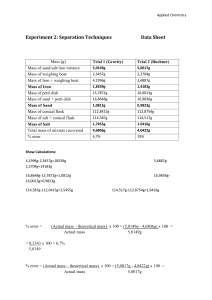
CP Chemistry Sludge Separation Experiment Lab 1.1 Name ________________ Date_______ Class _______ Purpose: Design and carry out a procedure to separate a mixture of sand, salt, water, iron and alcohol into it’s individual components. Calculate the % error of sand and Fe. Equipment: filter paper, funnel, magnet, Erlenmeyer flask, ring stand, iron ring, hotplate, electronic balance Procedure Hints: 1. Turn on hotplate and set to about 7. 2. Using the electronic balance mass approximately 5 g of sand. _______________ 3. Using the electronic balance mass approximately 8 g of Fe. _______________ 4. Add the sand and Fe to the container containing the rest of the mixture. 5. Begin separation process. 6. Thoroughly and carefully dry Fe and sand on the hotplate, don’t scorch the filter paper. 7. After separating the Fe and sand determine the mass of each that has been recovered. 8. When heating the liquid filtrate, after about half of the liquid vaporizes, use a piece of filter paper as a lid to prevent splattering. Write out a detailed, step by step description of how you performed the experiment. Neatness counts. _________________________________________________________________________________________ _________________________________________________________________________________________ _________________________________________________________________________________________ _________________________________________________________________________________________ _________________________________________________________________________________________ _________________________________________________________________________________________ _________________________________________________________________________________________ _________________________________________________________________________________________ _________________________________________________________________________________________ _________________________________________________________________________________________ _________________________________________________________________________________________ _________________________________________________________________________________________ _________________________________________________________________________________________ _________________________________________________________________________________________ _________________________________________________________________________________________ _________________________________________________________________________________________ _________________________________________________________________________________________ _________________________________________________________________________________________ _________________________________________________________________________________________ _________________________________________________________________________________________ _________________________________________________________________________________________ _________________________________________________________________________________________ _________________________________________________________________________________________ _________________________________________________________________________________________ _________________________________________________________________________________________ Data and Results Tape a small, dry sample of the sand and salt and iron (using clear tape) in the space provided. Save samples of the pure alcohol and water so that the instructor can initial that you successfully separated the mixture. Samples Sand Salt Iron Teacher's initials Alcohol Water THOROUGHLY CLEAN ALL GLASSWARE WITH SOAP AND WATER, AND BE SURE TO DRY IT. RETURN ALL EQUIPMENT TO THE APPROPRIATE CABINET. Post – Lab Questions 1. The chemical formulas of iron, salt, and sand are Fe, NaCl and SiO 2, respectively. Are these substances elements or compounds are they homogeneous or heterogeneous explain? 2. Are any of the substances magnetic? Is magnetism a physical or a chemical property and is it intensive or extensive? Explain. 3. Which substance(s) dissolved in water? Is solubility a physical or chemical property? Explain. 4. Is the combination of salt, sand and iron a new compound or a mixture? Explain. 5. Describe the results of the filtration process. Which substance remained on the filter paper after filtration? 6. Is the liquid that passed through the filter paper a pure substance? Explain. 7. Which has a higher boiling point water or alcohol? Explain how you would know this. 8. Calculate the % error of the sand that was recovered. 9. Calculate the % error of the iron that was recovered.