Electrochemical Kinetics
advertisement

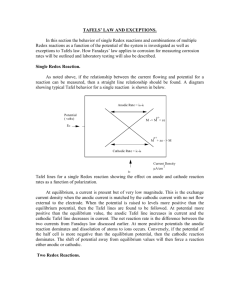
Lecture 3 Electrochemical Kinetics Basics Current density The rate of a homogeneous reaction, vr: vr 1 dn dt 1. where n and ν are the amount of material and stoichiometric number of a reactant or product. The unit for vr is mol s-1. Taking stoichiometric number to be one, vr dn dt The reaction rate of a heterogeneous process (e.g., an electrode process ) depends on the surface of the electrode at constant polarization potential. The rate v refers to unit surface area v 1 dn A dt 1 The unit for v is mol dm-2 s-1.The rate of an electrode process is proportional to the current density j 1 dq A dt The current density is proportional to rate of an electrochemical reaction and it makes rate independent of the area of electrode. An infinitesimal amount of charge dq is proportional to the amount of material passed through the interface dq zF dn . j zF dn A dt 2 From equations 1. and 2. we get j zF v 3 1 Lecture 3 The reaction rate of a unit surface electrode is proportional to current density. Polarization, overvoltage Polarization: the shift in the voltage across a cell caused by the passage of current, departure of the cell potential from the reversible (or equilibrium or nernstian) potential. For a simple reversible redox reaction Mz ze- M the cathodic process is a reduction at a rate jc, cathodic current density, while the anodic process is an oxidation at a rate ja, anodic current density. At equilibrium a rest or equilibrium potential εe is measured against a proper reference electrode. When electrochemical equilibrium is established j c j a j0 the anodic and cathodic current densities are the same and equals to j0, the exchange current density. Increasing the negative potential: jc j a Fig. 1. Polarization curves 2 Lecture 3 The portion of potential differs from equilibrium potential is called overvoltage (η ). pol e 4. Processes at electrodes The rate constant of forward reaction, kf is characteristic to the cathodic reduction process. The higher the negative polarization potential, the greater the rate of cathodic reaction. Fig. 2. Heterogeneous process at the electrode surface. Mass transport: reactants are transported from the bulk of solution to the electrode surface by diffusion products are transported from electrode to the bulk by diffusion Charge transfer: the electron jump between the metal and liquid phase. The slowest process determines the net rate of the electrode reaction. Mass transport and charge transfer are the most important steps in controlling the rate of electrochemical processes. 3 Lecture 3 Reaction mechanisms 1.Fast mass transport but rate limiting electron transfer kinetics. 2. Fast electron transfer but mass transport rate limiting kinetics Normal homogeneous kinetics at a temperature is characterized a rate constant independent of the composition of reaction mixture. Why can an applied voltage affect the kinetics of a reaction? Question can be answered at the level of equilibrium electrochemistry G o RT ln K a and G o nFE o Question can be answered at the level of electrochemical kinetics by using the transition state theory (TST) Consider a reduction reaction: O + ze- R By TST, the species, O with gain an electron and goes through a transition state. The energy barrier to forming this is called ΔG‡c. The c subscript denotes this to be a cathodic reaction – that is a reduction reaction. The free energy barrier of an electrochemical reaction is linked to the applied potential. 4 Lecture 3 G‡a(2) G‡c(2) G nFE G‡c G‡a O + neR Reaction coordinate When a potential is applied, the free energy of reactants (O + ne-) is raised by an amount zFE where E is the applied potential. When η = 0 Energy to reach transition state = ΔG‡c. When η > 0 New energy to transition state = ΔG‡c(2) The reaction proceeds faster when a negative polarization potential ε = εpol is applied. As a result: ΔG‡c(2) < ΔG‡c. The converse is true for the back reaction: R O + ne-. For the reduction reaction: ΔG‡c(2) = ΔG‡c + αnFε 5.a Remember: cathodic potential is negative. 5 Lecture 3 For the back reaction (oxidation of R) we can write: ΔG‡a (2) = ΔG‡a – (1-α)nFε 5.b The quantity α relates to the symmetry of the energy barrier. 0 < α <1 We can write the Arrhenius equation for the forward and back reactions: For forward (cathodic) reaction: Gc k f A f exp RT For backward (anodic) reaction: Ga k b Ab exp RT From 5.a and 5.b we get ΔG‡c = ΔG‡c(2) - αzFε and ΔG‡a = ΔG‡a(2) + (1-α)zFε So we can write: k f A f exp Gc zF exp RT RT Ga 1 zF kb Ab exp exp RT RT But since the first part of both equations are potential independent we do not need to consider them further and can write: k f k 0f exp zF 6.a RT 6 Lecture 3 k 0f That is: Gc A f exp RT and kb kb0 exp 1 zF 6.b RT Now we have two expressions that relate how the forward and back rate constants for an electron transfer reaction at an electrode are affected by the applied potential. The polarization curve for charge transfer limiting process, j = f(η) Recall Eq. 3: j I zF v A The rate of a homogeneous process for reaction O + ne- R, can be given v , k ,f cox and for a reaction at the electrode surface v 1 0 k f cox A 7. 0 where the rate refers to unit surface area, and cox is the concentration of oxidized form at the surface. From equations 3. and. 7. jc zF 1 0 k f cox A for a cathodic process 7 Lecture 3 ja zF 1 0 kb cred A for an anodic process The constant area of the electrode can be included in rate constants. The net current j can be given as j jc j a and 0 0 j zF k f cox kb cred The mass transport is fast, therefore the surface concentrations are identical to the concentrations in the bulk phase. Substituting rate constants from 6.a and 6b. 1 zF zF 0 0 j zF k 0f cox exp kb0 cred exp RT RT 8. This is the Butler-Volmer equation and very important in understanding electrode kinetics. Apart from thermodynamic activation, the exponential term shows us the potential dependence of rate constant. At equilibrium potential the cathodic and anodic reaction proceeds at an equal rate: 0 j0 zF k 0f cox exp zF 0 RT 0 zF kb0 cred exp 1 zF 0 RT The j = f(η) is called polarization curve, therefore equation 8. should be transformed From pol e we have, 1 zF e zF e 0 0 0 j zF k 0f cox exp kb cred exp RT RT 8 Lecture 3 which can be separated, (showing only for the cathodic part) zF zF e 0 j zF k 0f cox exp exp RT RT 9. Recognized in equation 9. the part of exchange current density: zF j j0 exp RT and 1 zF zF j j0 exp exp RT RT This is the equation of a polarization curve giving a current potential characteristics of an electrochemical process for a metal electrode of clean surface, crystal parameters, electrolyte composition concentration are known and the process is charge transfer controlled. Current vs overpotential curve, transfer coefficient α = 0.5 9