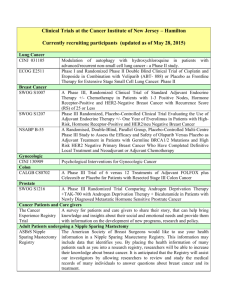
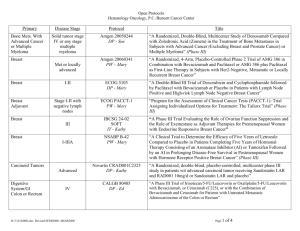
GOG-0219: A Phase III, Randomized Trial of Weekly Cisplatin and
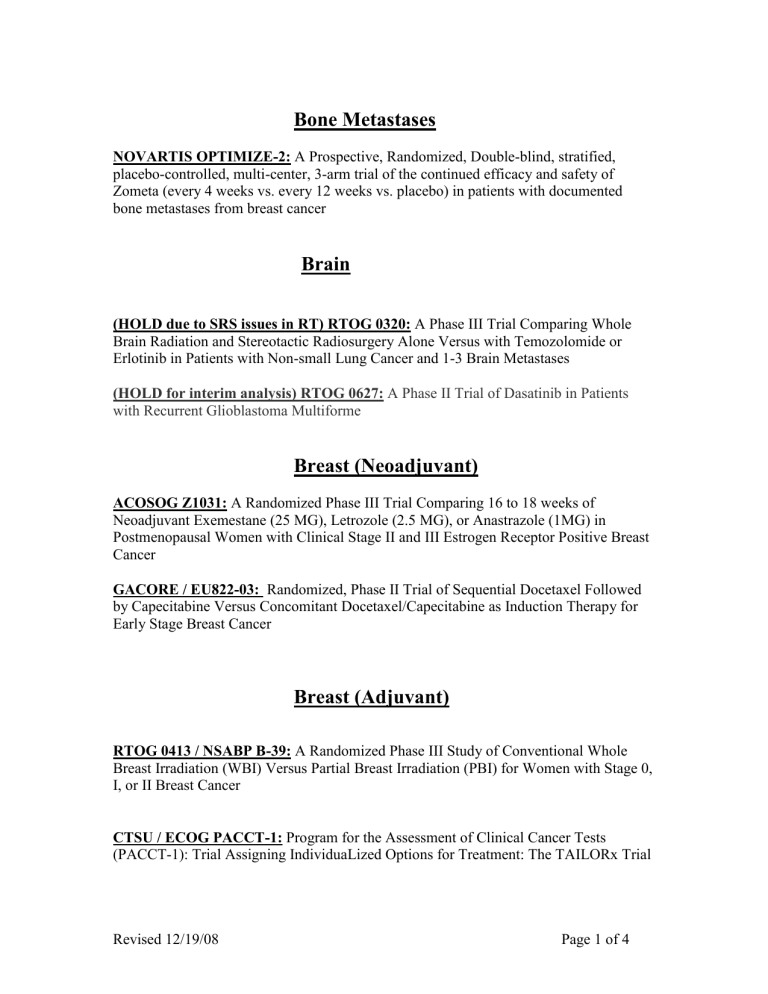
Bone Metastases
NOVARTIS OPTIMIZE-2: A Prospective, Randomized, Double-blind, stratified, placebo-controlled, multi-center, 3-arm trial of the continued efficacy and safety of
Zometa (every 4 weeks vs. every 12 weeks vs. placebo) in patients with documented bone metastases from breast cancer
Brain
(HOLD due to SRS issues in RT) RTOG 0320: A Phase III Trial Comparing Whole
Brain Radiation and Stereotactic Radiosurgery Alone Versus with Temozolomide or
Erlotinib in Patients with Non-small Lung Cancer and 1-3 Brain Metastases
(HOLD for interim analysis) RTOG 0627: A Phase II Trial of Dasatinib in Patients with Recurrent Glioblastoma Multiforme
Breast (Neoadjuvant)
ACOSOG Z1031: A Randomized Phase III Trial Comparing 16 to 18 weeks of
Neoadjuvant Exemestane (25 MG), Letrozole (2.5 MG), or Anastrazole (1MG) in
Postmenopausal Women with Clinical Stage II and III Estrogen Receptor Positive Breast
Cancer
GACORE / EU822-03: Randomized, Phase II Trial of Sequential Docetaxel Followed by Capecitabine Versus Concomitant Docetaxel/Capecitabine as Induction Therapy for
Early Stage Breast Cancer
Breast (Adjuvant)
RTOG 0413 / NSABP B-39: A Randomized Phase III Study of Conventional Whole
Breast Irradiation (WBI) Versus Partial Breast Irradiation (PBI) for Women with Stage 0,
I, or II Breast Cancer
CTSU / ECOG PACCT-1: Program for the Assessment of Clinical Cancer Tests
(PACCT-1): Trial Assigning IndividuaLized Options for Treatment: The TAILORx Trial
Revised 12/19/08 Page 1 of 4
CTSU / SWOG S0221: A Phase III Trial of Continuous Schedule AC + G Versus Q 2
Week Schedule AC, Followed by Paclitaxel Given Either Every 2 Weeks or Weekly for
12 Weeks as Post-Operative Adjuvant Therapy in Node-Positive or High-Risk Node-
Negative Breast Cancer. High-risk node negative patients with tumor > 2cm in greatest diameter. If patient is node positive a minimum of 6 lymph nodes must have been examined histologically.
CTSU / IBCSG 24-02: A Phase III Trial Evaluating the Role of Ovarian Function
Suppression and the Role of Exemestane as Adjuvant Therapies for Premenopausal
Women with Endocrine Responsive Breast Cancer
CTSU / SWOG S0307: Phase III Study of Bisphosphonates as Adjuvant Therapy in
Breast Cancer
CTSU / NSABP B-42: A Clinical Trial to Determine the Efficacy of Five Years of
Letrozole Compared to Placebo in Patients Completing Five Years of Hormonal Therapy
Consisting of an Aromatase Inhibitor (AI) or Tamoxifen Followed by an AI in
Prolonging Disease-Free Survival in Postmenopausal Women with Hormone Receptor
Positive Breast Cancer
CTSU / NCCTG N063D (ALTTO): A djuvant L apatinib and/or T rastuzumab
T reatment O ptimization Study: A Randomized, Multi-Center, Open-Label, Phase III
Study of Adjuvant Lapatinib, Trastuzumab, Their Sequence and Their Combination in
Patients with Her2/ErbB2 Positive Primary Breast Cancer
Breast (Advanced)
PFIZER A6181094: A Phase 3 Study of SU011248 in Combination with Paclitaxel
Versus Bevacizumab with Paclitaxel in the First-Line Advanced Disease Setting in
Patients Having Breast Cancer
SOPHERION STM01-102: A Phase III Randomized, Controlled Trial of Myocet,
Trastuzumab and Paclitaxel versus Trastuzumab and Paclitaxel for First line Therapy of
Metastatic Breast Cancer
CTSU / CALGB 40503: A Randomized, Double-Blind, Placebo-Controlled Phase III
Trial of Endocrine Therapy Alone or Endocrine Therapy Plus Bevacizumab (NSC
704865; IND7921) for Women with Hormone Receptor-Positive Advanced Breast
Cancer
CTSU/NCCTG N0733: A Randomized Phase III Trial of Capecitabine and Lapatinib with or without IMC-A12 in Patients with HER-2 Positive Breast Cancer Previously
Treated with Trastuzumab and an Anthracycline and /or a Taxane.
Revised 12/19/08 Page 2 of 4
Colon / Rectal / Anal Cancer
CTSU / ECOG E5202: A Randomized Phase III Study Comparing 5-FU Leucovorin and
Oxaliplatin versus 5-FU, Leucovorin, Oxaliplatin and Bevacizumab in Patients with
Stage II Colon Cancer at High Risk for Recurrence to Determine Prospectively the
Prognostic Value of Molecular Markers
(HOLD pending addendum)CTSU / CALGB-80405: A Phase III Trial of Irinotecan /
5-FU / Leucovorin or Oxaliplatin / 5-FU / Leucovorin with Bevacizumab, or Cetuximab
(C225), or with the Combination of Bevacizumab and Cetuximab for Patients with
Untreated Metastatic Adenocarcinoma of the Colon or Rectum
(HOLD pending addendum)CTSU / SWOG S0600: A Phase III Trial of Irinotecan-
Based Chemotherapy Plus Cetuximab (NSC-714692) with or without Bevacizumab
(NSC-704865) as Second-Line Therapy for Patients with Metastatic Colorectal Cancer who have Progressed on Bevacizumab with either FOLFOX, OPTIMOX or XELOX
CTSU / ECOG E5204: Intergroup Randomized Phase III Study of Postoperative
Oxaliplatin, 5-Fluorouracil and Leucovorin vs Oxaliplatin, 5-Fluorouracil, Leucovorin and Bevacizumab for Patients with Stage II or III Rectal Cancer Receiving Pre-operative
Chemoradiation
CTSU / NSABP R04: A Clinical Trial Comparing Preoperative Radiation Therapy and
Capecitabine with Preoperative Radiation Therapy and Continuous Intravenous Infusion
(CVI) of 5-Fluorouracil (5-FU) in the Treatment of Patients with Operable Carcinoma of the Rectum (Note: Endoluminal Ultrasound is required within 42 days prior to registration – Dr. William Taylor is the only physician in town to do this procedure)
Gynecological Oncology
CTSU / GOG 0209: A Randomized Phase III Trial of Doxorubicin/Cisplatin/Paclitaxel and G-CSF Versus Carboplatin/Paclitaxel in Patients with Stage III & IV or Recurrent
Endometrial Cancer
GOG-0212: A Randomized Phase III Trial of Maintenance Chemotherapy Comparing
12, Monthly Cycles of Single Agent Paclitaxel or Xyotax (CT-2103) Versus no
Treatment until Documented Relapse in Women with Advanced Ovarian or Primary
Peritoneal Cancer who Achieve a Complete Clinical Response to Primary
Platinum/Taxane Chemotherapy
Revised 12/19/08 Page 3 of 4
GOG-0219: A Phase III, Randomized Trial of Weekly Cisplatin and Radiation Versus
Cisplatin and Tirapazamine and Radiation in Stage IB2, IIA, IIB, IIIB and IVA Cervical
Carcinoma Limited to the Pelvis
Lung
CTSU / ECOG E1505: A Phase III Randomized Trial of Adjuvant Chemotherapy with or without Bevacizumab for Patients with Completely Resected Stage IB (
4 cm) – IIIA
Non-Small Cell Lung Cancer (NSCLC)
Lymphoma
NO TRIALS CURRENTLY OPEN
Prostate
RTOG 0521: A Phase III Protocol of Androgen Suppression (AS) and 3DCRT/IMRT
VS AS and 3DCRT/IMRT Followed by Chemotherapy with Docetaxel and Prednisone for Localized, High-Risk Prostate Cancer
Renal
CTSU / ECOG E2805 : A Randomized, Double-Blind Phase III Trial of Adjuvant
Sunitinib versus Sorafenib versus Placebo in Patients with Resected Renal Cell
Carcinoma
CTSU/ ECOG E2804: The BeST Trial: A Randomized Phase II Study of VEGF, RAF
Kinase, and mTOR Combination Targeted Therapy (CTT) with Bevacizumab, Sorafenib, and Temsirolimus in Advanced Renal Cell Carcinoma
Revised 12/19/08 Page 4 of 4