chemical hygiene plan - Chemistry at Winthrop University
advertisement

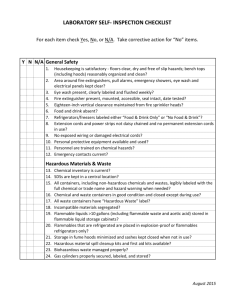
SECTION 4 CHEMICAL HANDLING AND STORAGE REQUIREMENTS A. DEFINITIONS AND WARNING LABELS. 1. Definition of chemical hazards. a. Explosives-Chemicals that cause a sudden, almost instantaneous release of pressure, gas, and heat when subjected to sudden shock, pressure, or high temperature. Example-fireworks, nitrogen triiodide, gunpowder b. Flammable liquid-Any liquid having a flash point below 100F (38C). This class is further subdivided into: IA. Flash point below 73. (23C) Boiling point below 100 (38C) Example: pentane, Tetrahydrofuran, Ethyl ether, Carbon disulfide IB. Flash point below 73F (23C) Boiling point at or above 100oF (38C) Example: Acetone, Ethyl acetate, Ethanol, Triethylamine, Toluene. Methyl Ethyl Ketone IC. Flash point 73F (23C) to 100F (38C) Example: Xylene, Butanol, Turpentine c. Combustible liquid: Flash point 100F (38C) to 200F (93C) Example: acetic acid, Kerosene d. Flammable solid: solid other than an explosive that is liable to cause fire through absorption of moisture or chemical change, or which can easily be ignited and burns vigorously and persistently. Example: Benzoyl Peroxide, Calcium Carbide, Picric acid e. Toxic Chemical: Any chemical that has been shown to cause acute toxic or severe chronic health effects in exposed workers. This would include acute toxins, suspect carcinogens, and mutagens. Example: Bromine, Hydrofluoric Acid, Phosgene, and Nicotine f. Select Carcinogen: Any chemical which is regulated by OSHA as a carcinogen, or listed under "Known Carcinogen" by the National Toxicology Program (NTP), or is listed under human carcinogens by the International Agency for Research on Cancer (IARC). Example: Asbestos, Vinyl Chloride, Benzidine g. Cryogen: Liquefied gases that condense oxygen from the air, create an oxygen rich atmosphere and increase the potential for fire if flammable or combustible materials and a source of ignition are present. h. Etiologic Agent: A viable microorganism or toxin, which causes or may cause human disease. i. Radioactive Material: Any material or combination of material which spontaneously emits ionizing radiation having specific activity greater than 0.002 microcuries per gram. Example: Uranium 235 j. Permissible Exposure Limits (PELs): The average amount of any chemical vapor that workers can be exposed to during an eight hour day. Example: the PEL for Benzene is 30 mg/cubic meter. k. Threshold Limit Values (TLVs): The time weighted average concentrations for a normal eight hour workday and a 40 hour work week, to which nearly all workers may be repeatedly exposed, day after day, without adverse effects. 2. LABELS a. All chemicals will have their manufacturer's original container warning label about hazards. b. Storage containers that do not have a manufacturer's label will be labeled by the Chemistry Department with a computer label. c. For smaller working amounts of chemicals that are transferred to secondary containers, those containers must be properly labeled including any health hazards. The container must be labeled with: -The contents of the container i.e. the common name of the chemical. Chemical formulas and structural formulas are not acceptable except for small quantities of compounds synthesized in the laboratory. -Physical and health hazards This labeling requirement does not apply to portable containers intended for the immediate use by the employee or student performing the transfer and to students assigned unknown chemicals for analysis. However, hazard information should be provided with all unlabeled chemicals in student labs. d. DOT labeling system There are also Department of Transportation (DOT) labels that are used on external packaging of all shipments of chemicals and thus should be understood by all employees. DOT labels use color-coded diamonds with picture and word warnings to convey their messages. They are largely self-explanatory. B. PROCUREMENT AND RECEIPT OF CHEMICALS 1. Incoming chemicals: Requisitions for chemicals are initiated by faculty members or the lab manager. Chemicals are delivered to the supply room or to the chemical storage building. 2. Check-out and labeling: When a shipment arrives, the lab manager will inspect the shipment to ensure that it is in fact the material ordered, is in good working condition, and that a MSDS is provided. Each container will be marked with the date of receipt and with an appropriate warning label. C. GENERAL HANDLING AND STORAGE CONDITIONS 1. No smoking or flames of any kind in chemical storerooms. 2. A nonbreakable, secured secondary container should be used for transporting any hazardous chemical. 3. All storage rooms shall have continuoes ventilation and must be checked if any buildup of odors is noticed. 4. Annual inspections of all containers for seal, label integrity, warning labels, quantity on hand, and any signs of decomposition. 5. Storage of chemicals in hoods and on lab benches is discouraged and all such containers shall be returned to the appropriate storage area whenever the experiment is complete. 6. Aisles in storage rooms will not be blocked. 7. Keep chemicals away from heaters and sunlight. 8. Ensure storerooms do not have floor drains in order to prevent contamination of water supplies. 9. A storeroom shall be clearly posted for the type of hazards inside. 10. All refrigerators used for the storage of potentially explosive materials must be explosion proof. Clearly label all materials placed in refrigerators. All refrigerators must be labeled to indicate its general use. For example: Chemical storage only. Do Not Store Food In This Refrigerator. Additionally, all non-explosion proof refrigerators will be labeled. Such as Do Not Store Flammable Materials In This Refrigerator. D. FLAMMABLE CHEMICAL STORAGE 1. Containers of chemicals with flashpoints less than 200 F and one gallon or larger containers shall be stored in the flammable storage room of the chemical storage building. 2. All containers of chemicals with flash points less than 100 F shall be stored in a flammable storage cabinet. The size and number of such containers will be kept to a minimum. 3. Large amounts of flammable chemicals should be used only in vented hoods and away from sources of ignition. 4. Smaller working amounts of flammable chemicals should be used in vented hoods whenever possible and away from sources of ignition. 5. The quantities of flammable chemicals stored in the laboratories should be kept to minimum unless they are stored in a flammable storage cabinet. 6. Flammables should not be stored in areas exposed to direct sun light. 7. Heat flammable substances in steam, water, oil, hot air baths or heating mantles only. 8. Isolate all flammables from: 9. Appropriate fire extinguishers and/or sprinkler systems and spill control materials will be available in all areas where flammable chemicals are stored. 10. All chemical storage rooms will have a raised area in the doorway to contain spills. E. 1. 2. 3. PEROXIDE-FORMING CHEMICALS Date all peroxidizableles upon receipt and upon opening. Store away from heat and light sources. Potential peroxide formers such as ethyl ether, cyclohexane, and tetrahydrofuran will not be allowed to evaporate to dryness. 4. After the recommended disposal date, test the chemical for peroxide or dispose of properly. 5. Ethyl ether will not be kept for more than two years in any case. E. CORROSIVE CHEMICALS 1. Inorganic corrosives will be stored in acid room of the chemical storage building by themselves in a clearly labeled area. 2. Organic corrosives will be stored in a separate area of the organic storeroom and clearly labeled as such. 3. Keep storage areas dry, well ventilated and cool, but not cold as acetic acid freezes at 60F (16C) 4. Isolate corrosives from all other nearby chemicals. 5. Whenever possible store corrosives in their original shipping containers. 6. Acid spill control material will be readily available. 7. Store corrosives four feet or less above the floor. 8. Recognize that some acids, such as Perchloric and fuming Nitric, must be treated as strong oxidizers rather than acids. 9. Separate corrosives that will react with other corrosives. G. WATER REACTIVE CHEMICAL STORAGE 1. Isolate from other chemicals and label clearly. 2. Store in a dry place. H. COMPRESSED GAS STORAGE 1. All extra gas cylinders will be located in the chemical storage building. They will be secured at all times. Caps will remain on until in use. 2. Paper tags will be used on all cylinders to indicate the state of the tank as: Full, In Use, or Empty. 3. Cylinders will be transported on gas cylinder carts, with caps in place. 4. All cylinders outside the storage area must be securely attached to walls or benches with chains or straps. I. TOXIC CHEMICAL STORAGE All toxic chemicals will be stored in a separate area with appropriate warning signs. J. SELECT CARCINOGENIC CHEMICAL STORAGE 1. Isolate from all other chemicals and label the area clearly for the hazard involved. 2. Store close to the ventilation intake to minimize vapor hazards. 3. Dispose of as soon as possible. 4. See complete list in Appendix . K. CRYOGEN STORAGE 1. Always wear safety glasses with side shields or goggles when handling. Gloves should be impervious and sufficiently large to be readily thrown off should a cryogen spill. 2. Watches, rings, and other jewelry should not be worn 3. Containers and systems containing cryogens should have pressure relief mechanisms. 4. Containers and systems should be capable of withstanding extreme cold without becoming brittle. L. WASTE DISPOSAL if 1. Label all waste using a waste label that includes the name of the chemical, the concentration possible, the date collected, and the lab instructor’s name or the course where the waste came from. 2. Waste containers shall be transported the waste collection site in the chemical storage building. 3. Waste chemicals shall not be stored or left in hallways for future pickup.