Chemistry of Life
advertisement

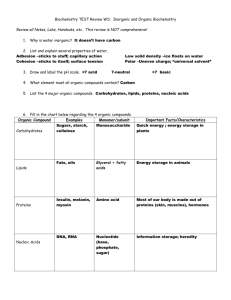
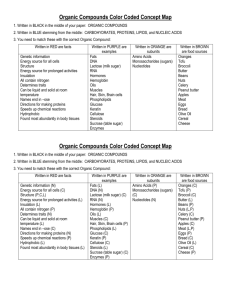
Study Guide Chemistry of Life: ELEMENTS 1. What is an element? 2. What are the most common elements in living things? 3. What does the Periodic Table of Elements represent? You should be able to recognize what number represents the atomic numbers, atomic weights and their abbreviations. 4. What is the abbreviation of an element called? (You should know that this is called a symbol. You should also know that some of the common elements such as oxygen = O, carbon = C, hydrogen = H.) Some symbols are written with a capital letter and others are written with a capital and a lowercase letter. Examples: C + Carbon; Co + Cobalt. 5. What is the smallest unit of an element called? 6. What are the three types of subatomic particles that make up an atom? 7. Which two subatomic particles are found in the nucleus? What type of charge does it have: positive, negative or neutral? 8. Which subatomic particle is found in a cloud surrounding the nucleus? What is the charge carried by these particles? 9. What is the name of the model given to describe the atom? 10. What is the atomic mass (weight) of an element? 11. Which element is found in most every living thing? COMPOUNDS: 1. What is a compound? 2. Give me an example of a compound? 3. What is the smallest unit of a compound? H2O is the formula for a molecule of water. This means a molecule of water consist of 2 atoms of hydrogen and 1 atom of oxygen. (You should be familiar with other common compounds such as CO2 (carbon dioxide) and NaCl (salt). 4. What are compounds that do not contain carbon called? (For example: carbon dioxide, which contains carbon but is not an organic compound. Water and salt are also inorganic compounds.) 5. What are the most important groups of organic compounds found in living things? 6. Where do these organic compounds come from? 7. Sugars and starches are what type of compound? 8. Why are carbohydrates important to cells? 9. What large organic molecules are made up of carbon, hydrogen, oxygen, nitrogen, and, in some cases, sulfur and found in foods such as meats, eggs, fish, nuts, and beans. 10. Why are proteins important to the cell? 11. What are the basic building blocks of protein called? How many are commonly found in most every protein? 12. What types of proteins are responsible for speeding up chemical reactions in living things? 13. What type of energy rich organic compounds are made of carbon, hydrogen, and oxygen and contain even more energy than carbohydrates? 14. Give an example of a type of lipid that is made in the liver and can be harmful if you eat too many eggs and/or if too much is found in your bloodstream? 15. What are very large organic molecules made of carbon, oxygen, hydrogen, nitrogen, and phosphorus that also contain the instructions that cells need to carry out all of life’s activities. 16. Name the two types of nucleic acids are found in the cell? 17. What is DNA? 18. What is RNA? WATER: 1. What percentage of water are humans made of? 2. Name four important ways cells use water. CELL PROCESSES: 1. What is the term when molecules go from an area of high concentration to an area of low concentration? 2. What are the three processes that cells use to move substances in and out, across the cell membrane? 3. Give me two examples of passive transport. 4. What is active transport? 5. What is osmosis? 6. Where does the water inside a cell move to, if the cell is in a salt solution? Why? 7. Where does the water inside a cell move to, if the cell is in a fresh water solution (a solution with a very low concentration of salt)? Why? 8. What does the cell need to move a substance across its membrane, against its concentration gradient? 9. Name the two types of active transport. 10. Where are transport proteins located and what do they do? 11. What is endocytosis? Study Guide (ANSWER KEY) ELEMENTS: 1. The most common elements in living things are carbon, hydrogen, oxygen, and nitrogen (C,H,O,N). 2. The Periodic Table of Elements is a chart that shows all of the elements known. The table is organized to show their atomic numbers, how elements are related, their atomic weights, their abbreviations and other information. 3. Each element has an abbreviation called a symbol. (You should know the symbols of some of the common elements such as oxygen = O, carbon = C, hydrogen = H.) Some symbols are written with a capital letter and others are written with a capital and a lowercase letter. Examples: C + Carbon; Co + Cobalt. 4. The smallest unit of an element is called an atom. An element is made up of only one kind of atom. The word atom comes from the Greek word, atomos, which means indivisible. 5. Atoms consist of three types of subatomic particles: protons, neutrons, and electrons. 6. Protons are found in the nucleus and have a positive charge. Neutrons are also found in the nucleus and have no charge. Most of the mass of an element is found in the nucleus. 7. Electrons are found in orbits called shells surrounding the nucleus. The electron shells form a “cloud” around the nucleus. Electrons have a negative charge and have very little mass. 8. This model is usually called the Electron Cloud Model. The atomic number of an element is the number of protons it has. 9. Carbon is the element found in most all living things. COMPOUNDS: 1. When two ore more different elements combine by sharing or donating electrons they form a compound. 2. Water is an example of a compound. 3. The smallest unit of a compound is a molecule. H2O is the formula for a molecule of water. This means a molecule of water consist of 2 atoms of hydrogen and 1 atom of oxygen. (You should be familiar with other common compounds such as CO2 (carbon dioxide) and NaCl (salt). 4. Many of the compounds found in living things contain the element carbon, which is usually combined with other elements. 5. Most compounds that contain carbon are called organic compounds. Compounds that do not contain carbon are called inorganic compounds. (The exception to this definition is carbon dioxide. It contains carbon but is not an organic compound. Water and salt are also inorganic compounds.) 6. The most important groups of organic compounds found in living things are: carbohydrates, lipids, proteins, and nucleic acids. 7. These organic compounds are found in the food we eat. The food we eat comes from living things (plants and animal products). 8. Carbohydrates are organic compounds made of the elements carbon, hydrogen, and oxygen. Sugars and starches are examples of carbohydrates. 9. Carbohydrates supply energy. They also help form the cell membrane and cell wall. Fruits, vegetables, and grains are carbohydrates. When you eat carbohydrates your body breaks them down into simple sugars, which provide energy. 10. Proteins are large organic molecules made up of carbon, hydrogen, oxygen, nitrogen, and, in some cases, sulfur. Proteins are found on foods that are high in protein are meats, eggs, fish, nuts, and beans. 11. The body uses proteins for repair and growth. Proteins make up the organelles in cells. 12. Proteins are made up of smaller molecules called amino acids. Twenty different amino acids make up a large variety of the proteins used by living things. 13. An enzyme is a type of protein that helps make chemical reactions take place in a living thing. For example, the enzymes in your saliva speed up the digestion of food by breaking down starches into sugars in your mouth. 14. Lipids (fats) are energy rich organic compounds made of carbon, hydrogen, and oxygen. Lipids contain even more energy than carbohydrates. Cells store the energy in lipids for later use. 15. Cholesterol is a lipid. Cholesterol is an important ingredient in making cell membranes. The liver produces the cholesterol needed to make cell membranes. Excess cholesterol in foods can damage arteries. 16. Nucleic acids are very large organic molecules made of carbon, oxygen, hydrogen, nitrogen, and phosphorus. Nucleic acids contain the instructions that cells need to carry out all of life’s activities. 17. There are two kinds of nucleic acids, deoxyribonucleic acid and ribonucleic acid. These two nucleic acids are known as DNA and RNA. 18. DNA stores the genetic information that is passed on to future generations. 19. RNA is responsible for helping to make proteins. Water: 1. Water makes up about two-thirds of your body, or 75%. Water is vital to the functioning of your body. 2. Most chemical reactions that take place in cells can only occur when the substances are dissolved in water. Without water most chemical reactions within cells could not take place. Water also helps cells keep their shape and size. Water also keeps the temperature of cells from changing too fast. Water helps carry substances into and out of cells. CELL PROCESSES: 1. Substances can move into and out of a cell by diffusion, osmosis, and active transport. 2. Diffusion is when molecules go from an area of high concentration to an area of low concentration (many to few). 3. Osmosis is a special type of diffusion that involves water molecules. Osmosis is the movement of water from where there are many to where there are few (high concentration to low concentration) through a selectively permeable membrane. 4. Both diffusion and osmosis are examples of passive transport. Passive transport requires no energy to happen. 5. Active transport requires the cell to use energy. Two types of active transport: one needs transfer (transport) proteins and another is engulfing (endocytosis). 6. Transport proteins will “pick-up” molecules outside the cell and carry them in using energy. 7. Engulfing (endocytosis) requires that the cell membrane surrounds the particle and bring it into the cell. The cell membrane then pinches off and forms a vacuole in the cell.