Lecture W8 2-26
advertisement

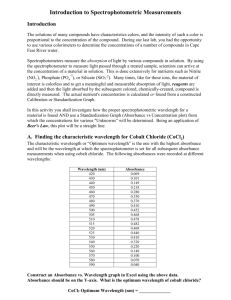
1 EA-AC Lecture W8 Today’s topics include: February 26, 2003 Spectrophotometer Detector Noise and Slit widths Analysis of Mixtures Photometric titrations Mole-ratio determinations of complexes Last Week we talked a bit about errors in spectrophotometers. Some sources of error are proportional to T. Cell positioning uncertainty falls in this class. Another problem is fluctuations in source intensity. If the light source doesn’t have constant output, then Io will vary with time and so will T and A measurements. XXXXXX Here are the results from the two different instruments we examined. The error of the Spec 20 is pretty much independent of transmittance. It is probable that the major source of uncertainty lies in the limited resolution of the transmittance scale that is read by eye. Errors that are independent of T include readout resolution, thermal detector noise – i.e. the noise in the detector when no light falls on it, and amplifier and other electronic noise. . _______ The Cary 118 does has particularly low error at high A. The errors that exist have their origin in the so-called shot noise that causes the outputs of photomultipliers and phototubes to fluctuate randomly about a mean value. XXXXX Shot noise is encountered whenever electrons cross a junction or a vacuum gap. In a photomultiplier, light strikes a photoelectric surface in a vacuum and electrons are emitted. These electrons are accelerated and strike a second plate that emits electrons – perhaps 6 of them. Each of these electrons are attracted to a third electron emitting plate where each produces 6 more electrons and so on. 69 = 10,077,696 The result is that a single photon produces a signal of about 107 electrons. The sum of all the electrons the are collected by the last plate per second become the current out of the detector and is proportional to the number of photons that strike the detector. But there is a randomness about the number of electrons emitted at each step. Thus the current is subject to statistical fluctuations , and these fluctuations vary with the square root of the current. All of this becomes unavoidable noise or error in the output of the spectrophotometer detector. 2 Now let’s revisit the idea of a slit width one more time. We said the wider the slit width the greater the spectral power that passes through it, but the larger bandwidth results in reduced spectral purity. So we have a design trade-off. XXXXX Suppose that this is the true power response to molecules of a particular substance. This theoretical curve is sometimes called the absorption envelope. Now let’s see how the spectrum might change with different spectral slit widths. Note especially the appearance of the narrow feature as the spectral slit width changes. The spectral slit widths are indicated by the distance between pairs of parallel lines on the wavelength axis as shown in this transparency. These lines bracket the range of wavelengths that pass through the monochromator at any nominal wavelength setting. The nominal wavelength is the center of the wavelength range. If the slit width is optimized, the spectrum will closely resemble the absorption envelope of this transparency. XXXXX If a narrower slit width is used, the decreased power arriving at the transducer means that the contribution from noise begins to obscure the spectral features. Here we see such a spectrum. All kinds of random noises now become visible. XXXXX If you are stuck with this situation, one possible approach is to signal average. Signal averaging simply involves obtaining the spectrum over and over again, adding the spectra together and dividing A at each wavelength by the number of spectra. XXXXXXX If the noise at each wavelength is truly random, then noise that is too high should balance noise that is too low. In general the noise is reduced by a factor equal to the square root of N the number of spectra taken. The HP diode-array has this feature built in by letting you vary how long to continue signal averaging spectra. The instrument takes a spectrum every 0.1 sec so collecting for 2 sec signal averages 20 spectra. Remember the transducer responds only to the total light power falling on it, with very little discrimination of wavelength. As a result, the transducer averages the power arriving at all the wavelengths passing through the monochromator. Consequently, as the monochromator scans through wavelength, when the edge of the slit reaches the spectral range of the sharp feature, the average power falling on the transducer rises. 3 This is seen as a rise in the power level before the monochromator's nominal wavelength reaches the base of the sharp peak. When the nominal wavelength of the sharp peak reaches the center of the slit, the highest point (the highest power) of the peak appears to be reduced, since the transducer is averaging the peak power with the points of lower power on either side. XXXXX As the slit is made wider than the optimum, the spectral width becomes greater than that of the sharp feature, and the sharp peak appears to be even broader and lower. Notice that the broad peak appears relatively unchanged through all the slit-width changes. The difference in the slit's effect on the broad and narrow features illustrates a general rule. The effect depends on the ratio of the linewidth of each spectral feature compared with the spectral slit width. As a general rule, in order to measure a spectral feature precisely, spectral bandwidth need to be < 1/10th the linewidth of the feature. As an example of the effect of slit width on the absorption spectrum of a real substance, here is the spectrum of reduce cytochrome c as the bandpass is varied from 20 nm to 1 nm. Here we see both peaks improve as we go to narrower slits. XXXXX Here is an example of the use of a spectrophotometer in analysis of a nickel complex. A 5.00 x 10-4 M solution of a nickel complex is put into a sample cuvette with a pathlength of 1.000 cm. The absorbance at 592 nm is found to be 0.446. What is the molar absorptivity of the nickel compound at that wavelength? To find ε592: A = ε592 b c 0.446 = ε592 (1.000 cm) (5.00 x 10-4 M) ε592 = 0.446/(5.00 x 10-4) = 8920 If a solution of unknown concentration of the nickel complex has an absorbance of 0.125 at the same wavelength, what is its concentration? A = ε592 b c 0.125 = 8920 x 1.000 c c = 0.125/8920 = 1.40 x 10-5 M So Beer’s law is based on a linear relationship between A and c. We can take advantage of this fact to analysis multiple chromophores in the same solution. 4 The total absorbance of a solution at any given wavelength is equal to the sum of the absorbances of the individual components in the solution. Atotal = A1 + A2 + …… + An = 1bc1 + 2bc2 +…… + nbcn. This relationship makes it possible in principle to determine the concentrations of the individual components of a mixture even if total overlap in their spectra exists. XXXXXX For example, this transparency shows the spectrum of a solution containing a mixture of species M and species N as well as absorption spectra for the individual components. Clearly, no wavelength exists at which the absorbance is due to just one of these components. At each point in the mixture the absorbance is Atotal = 1bc1 + 2bc2 To analyze the mixture, molar absorptivities for M and N are first determined at wavelengths 1 and 2 with enough standard to be sure that Beer's law is obeyed over an absorbance range that encompasses the absorbance of the sample. Note that the wavelengths selected are ones at which the two spectra differ significantly. Thus, at 1 the molar absorptivity of component M is much larger than that for component N. The reverse is true for 2. Once we know values for each substance at each of the two wavelengths then the absorbance of the mixture is determined at the same two wavelengths. A1 = 1bc1 + 2bc2 A2 = 3bc1 + 4bc2 We know everything except the two concentration terms, so we have two equations and two unkowns to solve for. You worked one of these problems in the homework and they are pretty straight forward. XXXX Here is a real world example of a two component solution. The top spectrum is of a CoEDTA solution while the middle spectrum is that of a Ni-EDTA solution, The bottom spectrum is from a mixture of the two solutions but not a 1:1 mixture. Using this method, you do not have to do an EDTA titration in order to do 3 sig fig analysis of a mixture of Co and Ni. Once you have determined the two ’s, you can do this analysis rapidly. 5 Our book presents in Chapter 19 an Excel spreadsheet method for solving these multicompoent mixture probems. Solving sets of linear equations is the basis for the math field called linear algebra. The book mentions both matrix and determinant methods for these analyses as well. Here is a graphical approach that is based on using more wavelengths to improve the statistics of the measurement. At any wavelength Am = A1s c2 A2s c2s A1s + c1 c1s Where the s’s represent values of the standard solutions and the rest are for the mixture. Notice that the equation has the form y = mx + b. This example is based on measurements at five wavelengths. Finding the slope and intercept then allow you to solve for c1and c2 because c1 = intercept * c1s and c2 = slope * c2s The advantage of this method is that you can use least squares analysis to get the best straight line from the data and use least squares statistics to determine the errors in the values of c1and c2. We have been talking about how instruments work and how we use spectroscopy to determine the concentration of analytes in samples. Now let’s look at how spectrophotometers are used in titrations. In order to do a titration of course we need to be able to locate the equivalence point. The application of absorption measurements obviously requires that one or more of the reactants or products absorb radiation or that an absorbing indicator be present. A photometric titration curve is a plot of absorbance as a function of titrant volume. The absorbance value must be corrected for solution volume. That is, the solution becomes more and more dilute with added titrant. So the Absorbance has to be corrected back to simulate the initial volume. XXXXX If conditions are chosen properly, the curve consists of two straight-line regions with different slopes, one occurring at the outset of the titration and the other located well beyond the equivalence-point region. The end point is taken as the intersection of extrapolated linear portions of the two lines. This transparency shows typical photometric titration curves. 6 The graph in the upper left is the curve for the titration of a nonabsorbing species with an absorbing titrant that is decolorized by the reaction. An example is the titration of thiosulfate ion with triiodide ion. As the letters indicate, s = 0 p = 0 and the titrant is colored t >0. 2 S2O32+ == > S4O62+ + 2 eI3- + 2 e- == > 3 IThe titration curve for the formation of an absorbing product from colorless reactants is shown in upper-middle graph (b). The product is colored but the analyte and titrant are not. An example is the titration of iodide ion with a standard solution of iodate ion to form colored triiodide. The remaining figures illustrate the curves obtained with various combinations of absorbing analytes, titrants, and products. Notice that the equivalence points are estimated by extrapolation of each of the volume corrected lines. Volume correction is achieved by multiplying each observed absorbance by (V + v)/V, where V is the original volume of the solution and v is the volume of added titrant. In order to obtain titration curves with linear portions that can be extrapolated, the absorbing system(s) must obey Beer's law. Photometric titrations are ordinarily performed with a spectrophotometer or a photometer that has been modified so that the titration vessel is held in the light path. The instrument is set to a suitable wavelength and with radiation passing through the analyte solution to the detector. The instrument is adjusted to a convenient absorbance reading by varying the source intensity or the detector sensitivity. Ordinarily, no attempt is made to measure the true absorbance since relative values are perfectly adequate for end-point detection. Titration data are then collected without alteration of the instrument settings. The power of the radiation source and the response of the detector must remain constant during a photometric titration. Cylindrical cells are ordinarily used and stirring provided to get complete mixing. Care must be taken to avoid any movement of the vessel that might alter the length of the radiation path. Photometric titrations often provide more accurate results than a direct photometric determination because the data from several measurements are pooled in determining the end point. 7 When there are other absorbing species in the matrix during direct abosrbance measurements we are many time forced to use the method of standard addition. However other absorbing species do not usually interfere with a photometric titration since only a change in absorbance is being measured. One advantage of a photometric end point is that the experimental data are taken well away from the equivalence-point region. Consequently, the equilibrium constant for the reaction need not be as favorable as that required for a titration that depends upon observations near the equivalence point such as is required for indicator end points. For the same reason, more dilute solutions can be titrated. Photometric titrations has been applied to all types of reactions. For example, most standard oxidizing agents have characteristic absorption spectra and thus produce photometrically detectable end points. Although standard acids or bases do not absorb, the introduction of colored acid/base indicators permits photometric neutralization titrations. XXXXX The photometric end point has also been used to great advantage in titrations with EDTA and other complexing agents. This transparency illustrates the application of this technique to the successive titration of bismuth(III) and copper(II). Bismuth binds EDTA tighter (large formation constant) and so binds first to EDTA, then copper binds. At 745 mn, the cations, the reagent, and the bismuth complex formed in the first part of the titration do not absorb, but the copper complex does. Thus, the solution exhibits no absorbance until essentially all the bismuth has been titrated. With the first formation of the copper complex, an increase in absorbance occurs. The increase continues until the copper equivalence point is reached. Further reagent additions cause no further absorbance change. Clearly, two well-defined end points result. Now let’s turn to the use of spectrophotometry for determining the composition of complex ions in solution and for determining their formation constants. A major advantage of the spectrophotometric method is that you do not have to isolate the complex as a pure compound. The power of the technique lies in the fact that quantitative absorption measurements can be performed without disturbing the equilibria under consideration. Although most 8 spectrophotometric studies of complexes involve systems in which a reactant or a product absorbs, nonabsorbing systems can also be investigated successfully. The most common techniques employed for complex-ion studies are the method of continuous variations given in the book and the mole-ratio method. In the method of continuous variations, cation and ligand solutions with identical analytical concentrations are mixed in such a way that the total volume (and hence the total moles) of reactants in each mixture is constant but the mole ratio of reactants ( XL) varies systematically (for example, 1:9, 8:2, 7:3, and so forth). The absorbance of each solution is then measured at a suitable wavelength and corrected for any absorbance the mixture might exhibit if no reaction had occurred. The corrected absorbance is plotted against the volume fraction of one reactant, that is, Vm/(Vm + VL), where Vm is the volume of the cation solution and VL that of the ligand. XXXXX A typical plot is shown in this transparency. A maximum (or minimum if the complex absorbs less than the reactants) occurs at a volume ratio Vm/VL corresponding to the combining ratio of cation and ligand in the complex. In the Figure, Vm/(Vm + VL) is 0.33 and VL/(Vm + VL) is 0.66; thus, Vm/VL is 0.33/0.66, which suggests that the complex has the formula ML2. The curvature of the experimental lines in the Figure is the result of incompleteness of the complex-formation reaction. A formation constant for the complex can be evaluated from measurements of the deviations from the theoretical straight lines. The Mole-Ratio Method is a third method for determine combining ratios of ligands and metals. In the mole-ratio method, a series of solutions is prepared in which the analytical concentration of one reactant (usually the cation) is held constant while that of the other is varied. A plot of absorbance versus mole ratio of the reactants is then prepared. If the formation constant is reasonably favorable, two straight lines with different slopes are obtained. The two intersect at a mole ratio that corresponds to the combining ratio in the complex. XXXXX Typical mole-ratio plots are shown in this transparency. Note that the ligand of the 1:2 complex absorbs at the wavelength selected so that the slope beyond the equivalence point is greater than zero. 9 We deduce that the uncomplexed cation involved in the 1:1 complex absorbs because the initial point has an absorbance greater than zero. Formation constants can be evaluated from the data in the curved portion of mole-ratio plots. A mole-ratio plot may reveal the stepwise formation of two or more complexes as successive slope changes, provided the complexes have different molar absorbtivities and provided the formation constants are sufficiently different from each other. The Slope-Ratio Method This approach is particularly useful for weak complexes but is applicable only to systems in which a single complex is formed. The method assumes (1) that the complex-formation reaction can be forced to completion by a large excess of either reactant and (2) that Beer's law is followed under these circumstances. XXXXX Let us consider the reaction in which the complex MxLy is formed by the reaction of x moles of the cation M with y moles of a ligand L: x M + y L < == > MxLy Mass-balance expressions for this system are CM = [M] + x [MxLy] CL = [L] + y [MxLy] where cM and cL are the molar analytical concentrations of the two reactants. We now assume that at very high analytical concentrations of L, the equilibrium is shifted far to the right and [M] << x [MxLy]. Under this circumstance, the first mass-balance expression simplifies to cM = x [MxLy] [MxLy] = cM/x So Beer's law becomes, A1 = b[MxLy] = bcM/x A plot of absorbance as a function of cM becomes linear whenever sufficient L is present to satisfy the assumption that [M] << x[MxLy]. The slope of this plot is b/x. When cM is made very large, we assume that [L] << y[MxLy], whereupon the second mass-balance equation reduces to CL = y[MxLy] and 10 A2 = b[MxLy] = bcL/y Again, if our assumptions are valid, a linear plot of A versus cL is observed at high concentrations of M. The slope of this line is b/y. The ratio of the slopes of the two straight lines gives the combining ratio between M and L: b/x = y/x b/y These are some of the other uses of photometric methods in analytical chemistry besides straight analyte concentration determination. Homework Graphing – zero not a point Exam Classes on Wed Mar 5 will be in 1039. Read Chapter 21 on AA.