ISHC2015 0019
The 25th ISHC Congress –Santa Barbara CA Aug 23-28, 2015
!
Important notes:
You MUST use this template. If you don’t use it your abstract WILL be rejected.
Do NOT enter author and institution information on this form. You will be able to enter this information online when you submit the abstract.
Do NOT write outside the boxes. Any text or images outside the boxes will be deleted.
Do NOT alter this form by deleting parts of it or adding new boxes. Simply enter your information into the boxes. The form will be automatically processed – if you alter it your submission will not be processed correctly.
An un-sized >10point font is highly recommended for Chemdraw graphics
Save this file in .doc or .docx
format.
Title:
Lewis acid-catalyzed cyclization reactions of amides of ethenetricarboxylates
Abstract: (Your abstract must use Normal style and must fit into the box. Do not enter author details)
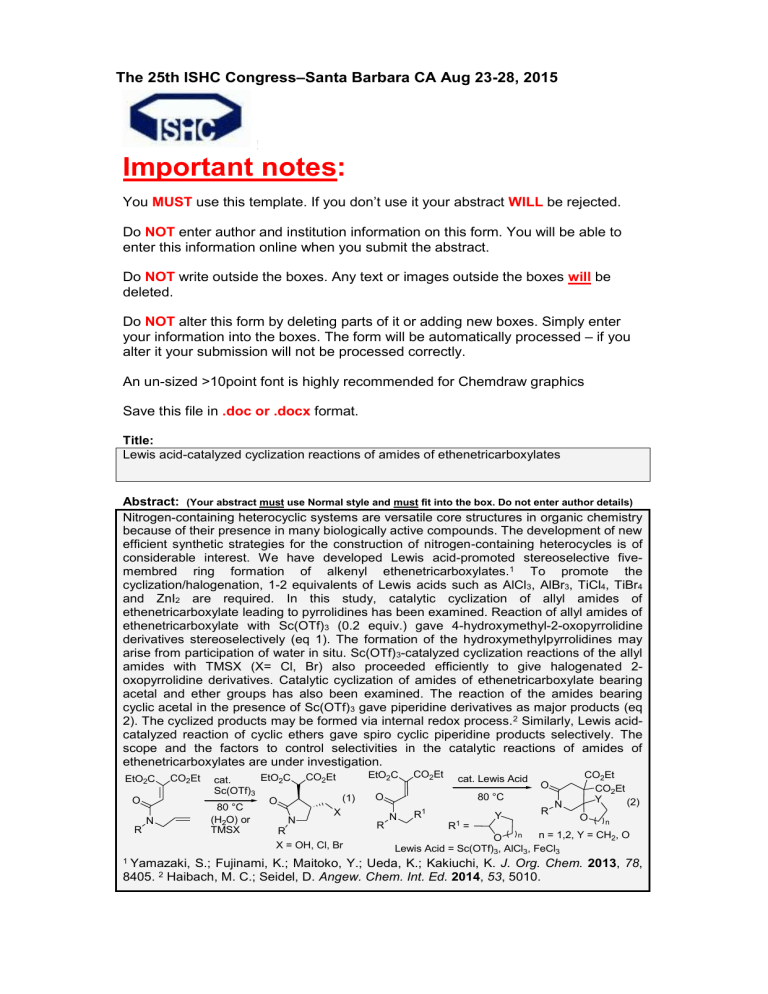
Nitrogen-containing heterocyclic systems are versatile core structures in organic chemistry because of their presence in many biologically active compounds. The development of new efficient synthetic strategies for the construction of nitrogen-containing heterocycles is of considerable interest. We have developed Lewis acid-promoted stereoselective fivemembred ring formation of alkenyl ethenetricarboxylates.
1 To promote the cyclization/halogenation, 1-2 equivalents of Lewis acids such as AlCl
3
, AlBr
3
, TiCl
4
, TiBr
4 and ZnI
2
are required. In this study, catalytic cyclization of allyl amides of ethenetricarboxylate leading to pyrrolidines has been examined. Reaction of allyl amides of ethenetricarboxylate with Sc(OTf)
3
(0.2 equiv.) gave 4-hydroxymethyl-2-oxopyrrolidine derivatives stereoselectively (eq 1). The formation of the hydroxymethylpyrrolidines may arise from participation of water in situ. Sc(OTf)
3
-catalyzed cyclization reactions of the allyl amides with TMSX (X= Cl, Br) also proceeded efficiently to give halogenated 2oxopyrrolidine derivatives. Catalytic cyclization of amides of ethenetricarboxylate bearing acetal and ether groups has also been examined. The reaction of the amides bearing cyclic acetal in the presence of Sc(OTf)
3
gave piperidine derivatives as major products (eq
2). The cyclized products may be formed via internal redox process.
2 Similarly, Lewis acidcatalyzed reaction of cyclic ethers gave spiro cyclic piperidine products selectively. The scope and the factors to control selectivities in the catalytic reactions of amides of ethenetricarboxylates are under investigation.
EtO
2
C CO
1
O
R
N
2
Et cat.
Sc(OTf)
3
80 °C
(H
2
O) or
TMSX
EtO
2
C CO
2
Et
O
R
N
X
X = OH, Cl, Br
(1)
EtO
2
O
R
C CO
2
N
R
1
Et cat. Lewis Acid
R
1
=
80 °C
Lewis Acid = Sc(OTf)
Y
O n
O
R
N n = 1,2, Y = CH
2
, O
3
, AlCl
3
, FeCl
3
CO
2
Et
O
CO
Y n
2
Et
(2)
Yamazaki, S.; Fujinami, K.; Maitoko, Y.; Ueda, K.; Kakiuchi, K. J. Org. Chem.
2013 , 78 ,
8405. 2 Haibach, M. C.; Seidel, D. Angew. Chem. Int. Ed.
2014 , 53 , 5010.