Laboratory 4 - Marks Group
advertisement

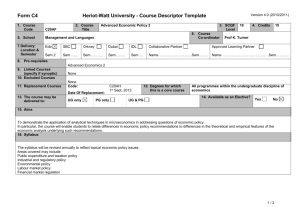
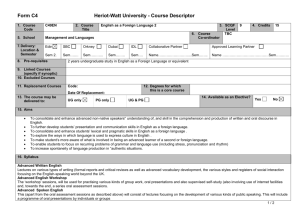
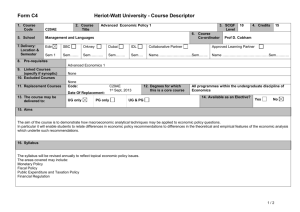
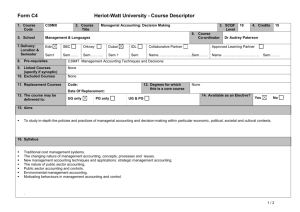
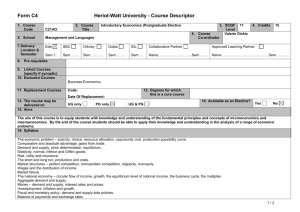
MAT_SCI 360 Introduction to Electron Microscopy Fall 2014 Laboratory 4: Introduction to SEM, Specimen Preparation, Condenser Lenses and Charging Introduction This laboratory is designed to introduce the Hitachi S-4800-II Scanning Electron Microscope. In this initial laboratory, the methods for basic operation and alignment of the instrument will be covered. You will also investigate methods for sample preparation, look at the effects of condenser lens strength on resolution and investigate the use of low kV to balance surface charge. The purpose of this lab is to get you familiar with the standard operation of the SEM and general facility practices. As you know from the course lectures, SEM provides the means to generate and analyze myriad signals generated from electron-specimen interactions. Of all these signals, the most commonly used imaging signal is the secondary electron (SE) signal. For this first laboratory, we will only be imaging with the SE signal in SEM, but we will explore other signals in the second laboratory. Learning Objectives: By the end of this laboratory session, you should be able to: 1. Understand how to prepare and mount both conductive and insulating samples for examination. 2. Start, load samples and align the Hitachi S-4800-II SEM and explain the effects of the alignment upon the imaging conditions 3. Increase the resolution of the SEM image through the use of the condenser lenses and explain this increase in terms of electron optics. 4. Balance surface charge on an electrically insulating sample by varying the accelerating voltage. Part I: Specimen Preparation In general, samples for the SEM must be both dry and electrically conductive. Since SEM imaging involves bombarding a material with an electron beam, the surface of the sample will accumulate charge if the electrons are not allowed to escape from the surface via a conductive path. If there is no such path, the image formed by the SEM will often be very poor, exhibiting imaging artifacts such as streaks, loss of contrast, distortion, etc. Charging can also lead to excessive heating of the sample, causing material degradation. Insulating and semiconducting materials (i.e. ceramics, polymers, and organics) should be coated with a conductive material to prevent surface charging specimens are typically coated with either a metal or carbon. Also, as the SEM is generally a high vacuum instrument, samples that contain water or other volatile components should be avoided as they may outgas in the chamber, which can cause imaging problems and potentially contaminate the chamber. EPIC also houses two variable pressure SEM (VP-SEM) instruments, which are capable of imaging non-conductive samples without coating. We won’t use the VP-SEM mode for these labs, but this may be useful for your projects or future research. As part of this laboratory, you will be introduced to some of the basic sample preparation techniques and equipment found in EPIC. The most important sample preparation equipment for 1 SEM includes the sputter coater, osmium coater, critical point drier and plasma cleaner. All of this equipment is available for use on your final projects. Part II: Starting up the Microscope Even though SEM's are expensive and technically advanced analytical tools, don't let them be intimidating. For the SEM on which you will be working, there are only a few ways in which you can damage the scope. If you remember the following things you will be fine: 1) Always make sure your sample holder is built correctly and the maximum height is not exceeded. 2) Make sure the samples are secured properly. If you have a magnetic sample, it needs to be very well attached to the stub. 3) Don’t use the exchange rod as a handle for the load lock door. 4) When in doubt, ask first! The Hitachi S-4800-II is a cold cathode field emission SEM. This means that the electron gun consists of a very sharp tungsten probe, which allows for a very high electric field concentration. This causes electrons to “tunnel” through the potential barrier, eliminating the need for thermal assistance. This has significant advantages for low kV operation. However, since the tip is so sharp, it is very sensitive to vacuum conditions and consequently there are separate ion pumps for the electron gun. Also, the tip must be “flashed” about once per day – this heats the filament and drives off any adsorbed molecules (the software will prompt you if flashing is necessary, otherwise do not flash). Part III: Aligning the Microscope Column In order to obtain good resolution it is essential that the microscope be properly aligned. Ideal alignment is achieved when the gun, lenses, and apertures are concentric about the optic axis - an imaginary line drawn down the center of the column. There are four basic procedures we need to complete, which are detailed in the manual: 1) 2) 3) 4) Align the electron gun Align the objective aperture Align the stigmators Correct for astigmatism Make sure you are comfortable with the alignment procedure as you will need to realign after each change in imaging conditions below. Part IV: Controlling Resolution with the Condenser Lens Since the SEM uses an electron probe to scan in the image of our sample point-bypoint, the diameter of the electron probe contributes to the resolution of that image. That is, the smallest feature we can resolve (image resolution) is limited by size of the electron beam at the sample (and the beam interaction volume). One way to control the diameter of the 2 electron beam, or probe size, is by manipulating the condenser lenses. The purpose of the condenser lenses is to demagnify the probe coming from the electron gun. The effect of the condenser lenses on the probe size is illustrated in the figure below (from Goldstein et al., 52): From the diagram, you can see that for a given working distance and objective aperture size, a stronger condenser lens will produce a smaller electron probe size, hence higher image resolution. However, you should also notice that the stronger condenser lens setting results in a lower probe current, indicated by the larger crosshatched area (more electrons are being “thrown away” from the beam). In electron imaging, this loss of probe current will be seen as a picture with more “snow” or “noise”. This is the trade-off that you will encounter over and over in using electron probe instruments - spatial resolution versus analytical sensitivity. To visualize these effects, take two different pictures of the same region on the sample (Au nanoparticles) with different condenser lens settings. Take 3 images with the condenser lens set to 1, 6 and 12. Use an accelerating voltage of 15kV, magnification >100,000X, working distance of <5mm and the Upper SE detector. 3 (Note: You will need to realign the column after each change to the condenser lens strength.) Part V: Controlling charging by varying the accelerating voltage We’ve already discussed various methods for reducing charging effects by applying conductive coatings to the sample. However, there are many situations where imaging the sample surface uncoated is preferred (e.g. if you want to continue processing of the sample and don’t want to alter the surface). In these situations, one is left with two options: use VPSEM or use low kV. Low kV operation has the advantage that it doesn’t require a special instrument, but also the images are more surface sensitive. In this part of the lab you will determine the E2 charge-balance point of a sample by varying the accelerating voltage and magnification. To do this, use the preset magnifications to quickly switch between high (10,000X) and low (1,000X) magnification. When you switch from high to low magnification, you will see a scan square which is either dark (positive charged) or light (negative charged). Change the accelerating voltage until you see little contrast difference between the scan square and the outside region. Make sure to move to new area each time. To complete this part of the lab, you will use a piece of a x386 processor that has been partially deprocessed. The device is encapsulated by an insulating layer which provides a good surface for the evaluation of the E2 point. As a starting point, the E2 point for most materials is 1-3 kV. You should capture quick images of both positive and negative charging as well as at the charge neutral condition. Laboratory Questions: In your laboratory report you should provide a description of all micrographs taken and concise discussion of differences you see. The handouts and your textbook should be sufficient for you to interpret your results, but you should provide an explanation in your own words. In addition to a discussion about the images, your lab report should provide answers to the following questions: 1) The cold cathode field emission gun has some benefits in terms of limiting chromatic aberration, etc. What are two major limitations of this type of electron gun for microanalysis (ie, EDS, WDS, etc.) compared to a Schottky thermal FEG or thermionic source? 2) When you turn the "focus" knobs on the SEM, what are you controlling in the microscope? If the objective aperture is misaligned and you change focus, what happens and why? (Hint: A diagram might be helpful) 3) In this lab we explored the effects of condenser lens strength on resolution and sensitivity. Changing the accelerating voltage also has an effect on both the resolution and sensitivity; however this relationship is generally not quite so straightforward. a) Probe size effects - In order to resolve small features in the SEM, one reasonable consideration is the ability to form a small probe. Discuss the general effect of increasing the 4 accelerating voltage on the minimum probe size you can form in the SEM. Let's say the smallest probe we can form in the 4800 SEM has a diameter of 2nm at 30kV, approximately what diameter will the smallest probe be at 3kV? 300V? (assume all other conditions are the same) b) Secondary electrons - The SE1 signal is the highest resolution signal in the SEM. Assuming the probe size is unchanged, what is the effect of changing accelerating voltage on the resolution of the SE1 signal? Assuming the probe current is unchanged, what is the effect of the accelerating voltage on the signal to noise ratio of the SE1 signal? c) Backscattered Electrons - The BSE and SE2 signals are important because they give rise to compositional contrast in the SEM. What is the effect of changing the accelerating voltage on the spatial resolution of these signals? 5) The image below is a pattern produced by electron beam lithography in PMMA on a silicon substrate. The PMMA was first spun onto the wafer (~150nm thick) and then the inner and outer lines of the letters in the ‘NUANCE’ pattern were written by taking control of the SEM scan coils. After exposure to the electron beam, the PMMA becomes more soluble in the developer and can be removed, leaving trenches around the letters. After developing, the sample was then sputter coated with a uniform thin film of Pt/Pd prior to imaging. For reference, the letters are ~2m tall and the lines are ~30nm wide. Why do the N, A and E have different contrast than the rest of the letters and the rest of the sample? Why are they dark? (This pattern was written several times on the sample and different letters were dark in each, so there is no difference in processing from letter to letter besides random variation.) Also, try to explain why the center of the ‘A’ is darker that the rest and why the ‘E’ is darker on the horizontal parts. 5 Tangibles: Laboratory report #1 is due one week after your laboratory session and is to be turned into the T.A. in the next laboratory session. Each group should hand in one laboratory report for all members. In laboratory reports you should be prepared to hand in the following: 1. Laboratory report, typewritten in standard laboratory report format including sections: Introduction, Procedure, Results and Discussion, Conclusion and References. The introduction should include motivations for the laboratory and background information on pertinent aspects of the laboratory (i.e., electron optical alignment, charging, etc.). It is not necessary to detail step-by-step operational procedures in the Procedure section, but instead discuss important steps in a more general or bullet form. Be concise and succinct. 2. Include all micrographs taken in this laboratory, appropriately labeled with detailed explanations in the Results and Discussion section of the report. The images captured with the 4800 SEM will be in a digital format, so please bring a flash drive to retrieve your data. The handouts and your textbook should be sufficient for you to interpret your results, but you should provide an explanation in your own words. 3. Answers to the questions at the end of these laboratory instructions. These can be included in the body of your report in the discussion section or as a separate section at the end. If you include them in the body of the report, please footnote to indicate which question you are answering. The report is due in one week after the lab. (or earlier). Submit via email to your TA and cc Professor Marks. References 1) Joseph I. Goldstein et al., Scanning Electron Microscopy and X-Ray Microanalysis, 2nd ed., (New York: Plenum Press, 1992). 6