Peter D. Kirkland, BVSc, PhD, FASM, PSM
advertisement

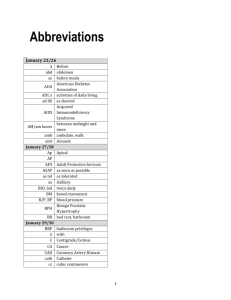
Clinical Signs, Epidemiology, Pathology and Diagnosis of an Emerging Teratogenic Vector Borne Viral Disease of Ruminants – Schmallenberg Virus as an Example Peter D. Kirkland Virology Laboratory, Elizabeth Macarthur Agriculture Institute, Woodbridge Rd, Menangle NSW Australia peter.kirkland@dpi.nsw.gov.au 1. Introduction Vector borne viruses that have been associated with teratogenic defects in ruminants have mostly belong to the Orthobunyavirus genus. The most well known is Akabane virus as a result of the large epidemics of congenital arthrogryposis and hyrdanencephaly (AG/HE) that have occurred, mostly in Australia and Japan but to a lesser extent in some Middle Eastern countries1 . Before the aetiological agent was recognised, a vector borne virus was suspected due to the geographical distribution of disease outbreaks and an association with favourable environmental conditions. However, there was some confusion about the linkage between a vector borne viral infection and the occurrence of disease due to the lack of disease in breeding females, the occurrence of disease in offspring during winter and spring months and the variety of defects that were observed. The recent emergence of Schmallenberg virus (SBV) presents another example of the issues that are faced when investigating disease outbreaks due to a similar novel agent. This presentation will focus on some of the important features of an incursion of a novel virus and the approaches to undertaking an effective investigation. Key elements involved in the investigation of a new agent such as SBV include: Appearance of an unusual clinical presentation/syndrome Rapid spread of disease over large geographical area Use of high throughput sequencing technologies for the identification of a novel agent Availability of archival sera to help identify time of the incursion Timely development of suitable assays for detection of virus and antibodies Application of new assays to detection of virus in animal tissues and insect vectors. 2. History and Clinical Signs The emergence of SBV has displayed, as time has progressed, most of the features of a typical orthobunyavirus. The most notable difference, that was fundamental to the discovery of this virus, was the occurrence of a transient febrile illness with a drop in milk production in infected cows. Within a short time frame, affected animals were observed on other farms, raising the question of a highly contagious or vector-borne disease. A very wide range of high consequence and exotic diseases were excluded2, including bovine ephemeral fever1. The application of high throughput sequencing and metagenomic investigations incriminated a virus from the Simbu serogroup2. As a consequence of the identification of a Simbu virus, the prospect of congenital defects in the progeny of infected pregnant animals was forecast and farmers and veterinarians advised of what might be seen. Some abortions were observed and several months later, commencing in early December, abnormal lambs were born, with a range of defects consistent with those described for other Simbu viruses. The incidence was not high in most flocks but in some instances, with synchronised ‘out of season’ breeding, up to 50% of lambs were affected. Towards the end of winter, nearing the end of the occurrence of cases in sheep, calves were born, firstly with arthrogyposis. These calves show varying locomotor difficulties and the limb defects are often the cause of dystocia. Later, animals affected with hydranencephaly and born. These calves are often stillborn or show severe CNS 1 deficits including blindness, deafness and extreme proprioceptive deficits. In cattle the proportion of affected calves was generally low. 3. Epidemiology While the initial cases of acute disease due to SBV on farms in The Netherlands and Germany near the Dutch/German border may not have raised suspicions of a vector borne virus, once the identification of a Simbu virus became known, vector transmission became the likely means of virus spread. Further, it was also likely that this novel virus would be spread by various Culicoides species. This is also supported by the speed of spread and the long distances over which affected farms were found in the first few months. In contrast, the radiating pattern of spread would, in the early stages, be potentially consistent with the introduction of a contagious agent into a fully susceptible population. It was soon recognised that SBV had emerged in a region close to where BTV serotype had emerged in Europe. This provided some indication of the potential vectors and the extent to which the virus might spread. At the start of the first winter, when a cessation to virus transmission was expected, both the occurrence of disease in adult cattle and, in most cases, serological evidence indicated that the virus had spread widely through The Netherlands, Germany, Belgium, Northern France and to England. In the subsequent years, spread has continued with infection also detected in Switzerland, Poland and several other Eastern European countries. Serological studies undertaken at the end of the first transmission season identified a very high seroprevalence in cattle – with evidence of infection on most farms and in many instances 80-90% of cattle. Surprisingly, there was also a relatively high prevalence in sheep, with up to 60% infected. Concern was expressed that the prevalence seemed too high for spread in a single season, based on transmission rates for BTV8 in similar locations. An alternative explanation proposed was that perhaps this virus was being spread by means other than just the vector – eg perhaps direct contact. The availability of archival serum collections facilitated the confirmation that SBV had recently arrived, with no evidence of infection before August 2011. The high transmission rates documented are consistent with knowledge of the annual incidence of infection with other Culicoides-borne Simbu viruses, even though the exact vector species may be different. The availability of rapid molecular diagnostic assays for the detection of SBV has also allowed stored insect collections to be studied and identify relatively high infection rates with SBV. These infection rates are markedly higher than encountered with BTV and provide some explanation for the apparently efficient transmission of virus to livestock. The available knowledge of local Culicoides species that have the capacity to act as vectors of other arboviruses (eg BTV) has also been valuable to explore the potential for virus transmission indoors and the likelihood of overwintering and a resumption of transmission early in the next season. Research undertaken in bulls has shown that SBV may be present in semen for a short period after the acute infection. Calf inoculation studies have confirmed that some samples contain infectious virus but it is not known whether this will establish infection in a female if used for insemination. 4. Pathology Both the gross and histopathological changes observed following SBV infection largely mirror those for virus such as Akabane3. The nature, severity of lesions and incidence of disease is determined by the host species, the stage of gestation at which foetal infection occurs and by the virus strain involved. The clinical presentation in cattle occurs in a sequential manner with a succession of different clinical signs and pathology at different 2 times after infection while in sheep and other small ruminants, a range of lesions and defects may occur in the same animal. With some strains of virus, modest numbers (15-20%) of animals may be affected while other strains may cause fewer defects, or in extreme situations, up to 80% of progeny have been affected. In most species, the impact of the virus is greatest in mid-gestation. There is no documented evidence of damage to the conceptus following infection in the very early stages of pregnancy (the first 3 weeks in sheep and goats; the first 2 months in cattle). The most susceptible stages of gestation in small ruminants range from 28-56 days (especially 28-36 days) and in cattle from 3 to 6 months. In the latter stages of gestation (after 60 days in small ruminants and the last 2 months in cattle), the incidence of abnormalities declines to a very low level. Naturally, during the course of an outbreak, the first abnormalities to be seen follow infection in the latter stages of gestation. Calves infected close to term may sometimes show a flaccid paralysis as a result of a non-suppurative encephalomyelitis. As infection occurs at earlier stages of gestation there may be arthrogryposis, perhaps only involving a single joint on a limb but after infection at earlier periods of gestation, limbs are more severely affected, with abnormalities involving multiple joints on several or even all four limbs. Arthrogryposis in cattle usually occurs as a result of infection between 100-170 days of gestation Infection of the foetus between approximately 80-105 days of gestation results almost exclusively in the development of hydranencephaly and porencephaly. There may be a small number of calves born with both mild hydranencephaly and arthogryposis due to infection around 100-120 days. The small grossly apparent cystic defects in the cerebral cortex (porencephaly) are seen in some calves with arthrogryposis but soon give way to calves that have severe CNS defects with almost complete absence of the cerebral hemispheres (hydranencephaly). In sheep and goats, due to the shorter gestation and shorter period of susceptibility, there is usually a combination of severe defects of the limbs concurrently with gross CNS lesions. There can be severe defects affecting the spinal column (torticollis, scoliosis, kyphosis, laudosis). Development of the lungs and thymus may also be retarded. The predominant lesions detected by histological examination are degenerative changes in the motor neurones of the spinal cord. These changes usually produce a neurogenic muscular atrophy but a primary viral myositis sometimes occurs. Histological examination of brains affected by hydranencephaly at term is usually unrewarding and reveals a thin shell of relatively normal tissue surrounding the large voids caused by viral replication. 5. Diagnosis Because it is not often observed, the occurrence of acute disease in adult animals over a large area cannot be relied upon to indicate the emergence of a novel teratogenic agent. However, the incursion of a virus into a susceptible population can be explosive and such signs may be noticed. Some strains of Akabane virus in Japan have caused clinical encephalitis in adult cattle. These may be key signs that later lead to severe outbreaks of congenital defects at a later time. If an epidemic of unusual disease is observed in an adult livestock population, to assist with an investigation, anticoagulant treated blood samples and serum should be collected from animals in the earliest detectable stages of the disease. These samples may be used for virus isolation or molecular based genome detection technologies. 3 Later in the season from midwinter extending to spring a teratogenic arbovirus should be considered when there is an outbreak of congenital defects in cattle, sheep or goats. An important factor to consider is the potential for contact with midges (Cucicoides spp) during the late summer and autumn. The gross pathology should provide a strong index of suspicion. Aetiological diagnosis and confirmation of an arbovirus infection depends on either the detection of the agent in tissues of the affected foetus or neonate or the detection of specific antibody in blood or fluids of affected progeny that have been deprived of colostrum. Most stillborn or aborted bovine foetuses and calves that are born at term mount a specific antibody response to the virus. The most conclusive approach to these investigations is to initially determine the IgG levels in foetal fluids (pericardial or pleural) or pre-colostral serum. An elevated IgG level will incriminate an infectious agent. Virus-specific serology can then be undertaken for known teratogenic viruses. However, low or ‘background’ IgG levels do not completely exclude the possibility of an infectious aetiology but serological methods will not be appropriate. Further investigations to exclude infectious agents will require cultural or nucleic acid detection methods. Virus detection by virus isolation or PCR may be considered from a foetus that has been aborted in the early stages of pregnancy. However, infectious virus is rarely present in full term neonates. Real time PCR has become a valuable diagnostic procedures and was employed to great effect during the investigation of the SBV outbreak. Viral RNA has been detected in tissues from a very high proportion of lambs at term. SBV virus was also detected in swabs of placenta and in meconium. In calves, residual viral RNA is occasionally detected at term, sometimes in conjunction with specific antibodies. Maternal serology can only be undertaken to exclude known agents and is of value only in regions where the virus is not endemic. In these situations, positive maternal serology will raise the index of suspicion while a negative result will convincingly exclude the test virus as the aetiological agent. During the investigation of a new or emerging vector-borne virus, once an isolate is available, or some of the genome sequence is known, it becomes possible to develop tests and further incriminate the agent. The development of qRT-PCR assays should be a priority to confirm involvement of the agent in the disease outbreak. Availability of the virus in culture is a key development but then allows the rapid development of serological tests. Basic assays such as the VNT or IFAT may used to detect specific antibody while other tests are being developed. 6. References There have been a considerable number of publications describing the Schmallenberg investigations. As these investigations are extensive, cover many countries and are complimentary, there are too many to accurately cite here, There is a recent review 4 that should be consulted to provide a detailed overview and a comprehensive list of relevant references. Several key papers are cited below. 1. Kirkland, P.D. 2002 Akabane and bovine ephemeral fever virus infections Vet Clin Food Anim, 18: 501-514. 2. Hoffmann, B., Scheuch, M., Hoper, D., Jungblut, R., Holsteg, M., Schirrmeier, H., Eschbaumer, M., Goller, K.V., Wernike, K., Fischer, M., Breithaupt, A., Mettenleiter, T.C., Beer, M., 2012. Novel orthobunyavirus in cattle, Europe, 2011. Emerging Infect. Dis. 18: 469-472 3. Kirkland, P.D., Barry, R.D., Harper, P.A.W. and Zelski, R.Z., 1988. The development of Akabane virus induced congenital abnormalities in cattle. Vet Rec, 122:582 586. 4 4. Tarlinton, R., Daly, J., Dunham, S. and Kydd, J. 2012. The challenge of Schmallenberg virus emergence in Europe. Vet. Journal. 194: 10-18 5