unit_2_mod_2_introduction_to_spectroscopy__uv
advertisement

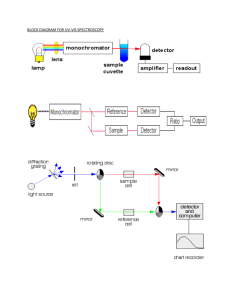
U2 M2 Intro. to spectroscopy & uv-vis spectroscopy page 1 of 10 Intro to Spectroscopy a) explain the nature of electromagnetic radiation b) know the approximate wavelength ranges of the X-ray, UV/VIS, IR and radiofrequency regions of the EM spectrum c) appreciate the relative energies and dangers associated with exposure to high energy wavelengths d) perform calculations using the equation E = hν = hc / λ e) appreciate that energy levels in atoms and molecules are quantised UV-VIS Spectroscopy a) explain the origin of absorption in UV/VIS spectroscopy b) explain why some species will absorb light in the uv/vis region but some others will not c) describe the basic steps involved in analysing samples by uv/vis spectroscopy (also mention use of complexing agents to form coloured compounds, as well as sensitivity and detection limits) d) state the Beer-Lambert Law and be able to use the equation to calculate the concentration of a given species in solution (implies the use of standards and calibration curves) e) list examples of the use of uv/vis spectroscopy in the quantitation of substance (refer to iron tablets, glucose and urea in blood, cyanide in water) Introduction to spectroscopy Electromagnetic radiation can be described in terms of a stream of photons, which are massless particles each travelling in a wave-like pattern and moving at the speed of light. Each photon contains a certain amount (or bundle) of energy, and all electromagnetic radiation consists of these photons. The electromagnetic (EM) spectrum is just a name that scientists give a bunch of types of radiation when they want to talk about them as a group. Radiation is energy that travels and spreads out as it goes-- visible light that comes from a lamp in your house and radio waves that come from a radio station are two types of electromagnetic radiation. Other examples of EM radiation are microwaves, infrared and ultraviolet light, X-rays and gamma-rays. Hotter, more energetic objects and events create higher energy radiation than cool objects. Only extremely hot objects or particles moving at very high velocities can create high-energy radiation like X-rays and gamma-rays. U2 M2 Intro. to spectroscopy & uv-vis spectroscopy page 2 of 10 EM waves are typically described by any three of the following physical properties: the frequency, , and wavelength, λ, and photon energy, E. Frequencies range from about a million billion Hertz (gamma rays) down to a few Hertz (radio waves). Wavelength is inversely proportional to the wave frequency, so gamma rays have very short wavelengths that are fractions of the size of atoms, whereas radio wavelengths can be as long as a several thousand kilometers. Photon energy is directly proportional to the wave frequency, so gamma rays have the highest energy around a mega electron volt and radio waves have very low energy around femto electron volts (femto = 10 − 15). These relations are illustrated by the following equation E = h variations below:or or Where: c = 3 x 108 m s-1 (speed of light in vacuum) & h = 6.626 ×10−34 Js-1 (Planck's constant). and its U2 M2 Intro. to spectroscopy & uv-vis spectroscopy Checkpoint A 1. 2. page 3 of 10 U2 M2 Intro. to spectroscopy & uv-vis spectroscopy page 4 of 10 High energy EM radiation e.g. ultraviolet rays, X-rays and gamma rays are very dangerous to living tissue. These energetic rays have to ability to disrupt and destroy DNA in tissue which can result in mutations or even death. All three types of rays would result in electronic transitions and even ionisation. In atoms, energy levels do NOT form a continuum. In fact, these energy levels are discrete or quantised. For example, energy levels may be at 5J, 10 J, 20 J but never 5.1J, 5.2 J, 10.3 J etc. There are no “in between” energy levels or values. UV-VIS Spectroscopy The absorption of ultraviolet or visible radiation corresponds to the excitation of outer electrons and thus electronic transitions. When an atom or molecule absorbs energy, electrons are promoted from their ground state (lowest energy level) to an excited state. Absorption of ultraviolet and visible radiation in organic molecules is restricted to certain functional groups (chromophores) that contain valence electrons of low excitation energy. Chromophores generally contain either П or lone pair(s) of electrons Electronic transitions generally seen are n* and * transitions. In essence molecules containing lone pairs of electrons OR electrons would absorb uv-vis radiation. All other transitions either do NOT occur or are TOO SMALL to be considered. Below is a diagram that shows the various electronic transitions. U2 M2 Intro. to spectroscopy & uv-vis spectroscopy Checkpoint B page 5 of 10 Underline the molecules in the list below which would absorb uv-vis radiation. O2, C6H6, NH3, CH4, C2H4, HCl Sample preparation UV-VIS samples are usually liquids or solutions. They are placed in a “cell” or cuvette which is a rectangular block, with two sides frosted and the other two sides clear and one end open and the other closed. The frosted sides can be touched but not the clear sides. Cells are made of fused synthetic silica. Solutions or liquids are placed in the cell in increasing concentrations. A “blank” (which contains everything else but the analyte in question) is first used to use as a reference point. Sometimes, complexing agents must be added to provide colour to the analyte which allows it to be analysed via uv-vis more easily. For example iron present in aqueous samples can be determined spectrophotometrically by complexation with a suitable complexing agent. The absorbance of the metal-ligand complex is usually measured in the visible region and is related to metal ion concentration. Colorimetric determination of iron can be done using several known complexing agents. Among the routinely used is 1,10-phenanthroline (phen) which reacts with Fe2+ to form an orange-red complex. Also dimethylglyoximeis is a complexing agent using to determine the amount of nickel present. The procedure depends on the construction of a calibration curve from standard Fe2+, followed by measurement of the unknown Fe2+ concentration from the curve. U2 M2 Intro. to spectroscopy & uv-vis spectroscopy page 6 of 10 Using absorbance to determine the concentration of an analyte The Beer-Lambert Law ε = molar absorbtivity ℓ = cell length in cm (usually 1 cm in length) c = molar concentration of solution A = absorbance Standards and calibration curves are used for UV-VIS spectroscopy coupled with Beer-Lambert Law as concentration of the analyte MUST show a linear signal response for the Law to be of any worth. NB Io = incident light, I = transmitted light Spectrophotometer The basic parts of a spectrophotometer are a light source, a holder for the sample, a diffraction grating or monochromator to separate the different wavelengths of light, and a detector. The radiation source is often a Tungsten filament (300-2500 nm), a deuterium arc lamp which is continuous over the ultraviolet region (190-400 nm), and more recently light emitting diodes (LED) and Xenon Arc Lamps for the visible wavelengths. The detector is typically a photodiode. Photodiodes are used with monochromators, which filter the light so that only light of a single wavelength reaches the detector. The dual-beam design greatly simplifies this process by simultaneously measuring P and Po of the sample and reference cells, respectively. Most spectrometers use a mirrored rotating chopper wheel to alternately direct the light beam through the sample and reference cells. The detection electronics or software program can then manipulate the P and Po values as the wavelength scans to produce the spectrum of absorbance or transmittance as a function of wavelength. Schematic of a dual-beam uv-vis spectrophotometer U2 M2 Intro. to spectroscopy & uv-vis spectroscopy page 7 of 10 Applications of UV-VIS spectroscopy UV/VIS spectroscopy is routinely used in the quantitative determination of solutions of transition metal ions and highly conjugated organic compounds, of iron in iron tablets, glucose and urea in blood and cyanide in water Solutions of transition metal ions which are too pale to give a reasonable absorbance can be complexed with various ligands to form more intense colours (i.e., absorb visible light) because d electrons within the metal atoms can be excited from one electronic state to another. The colour of metal ion solutions is strongly affected by the presence of other species, such as certain anions or ligands. For instance, the colour of a dilute solution of copper sulphate is a very light blue; adding ammonia intensifies the colour and changes the wavelength of maximum absorption (λmax) U2 M2 Intro. to spectroscopy & uv-vis spectroscopy Checkpoint C 1. page 8 of 10 U2 M2 Intro. to spectroscopy & uv-vis spectroscopy 2. page 9 of 10 U2 M2 Intro. to spectroscopy & uv-vis spectroscopy page 10 of 10 …………………………………………………………………………… ……………………………………………………………………………. …………………………………………………………………………… ……………………………………………………………………………. …………………………………………………………………………… ……………………………………………………………………………. …………………………………………………………………………… ……………………………………………………………………………. …………………………………………………………………………… ……………………………………………………………………………. …………………………………………………………………………… ……………………………………………………………………………. …………………………………………………………………………… ……………………………………………………………………………. …………………………………………………………………………… ……………………………………………………………………………. …………………………………………………………………………… ……………………………………………………………………………. …………………………………………………………………………… …………………………………………………………………………….