materials
advertisement

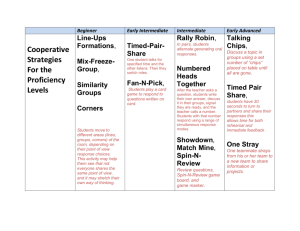
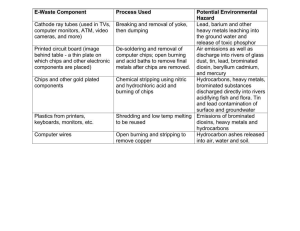
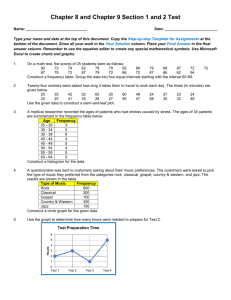
Name __________________________________ Period _____ Date ____________ The Density of Earth Materials SKILLS AND OBJECTIVES Measure the mass and volume of several objects. Compute and compare the density of these objects. Compare computed values with accepted values. Graph and interpret data. Density measures the amount of material (mass) in a given space (volume). The density of any earth material is the ratio of its mass to its volume, expressed as the D = m/V. In this lab, you will determine the density of two kinds of solids: those with regular geometric shapes (such as cubes or rectangular blocks) and those with irregular shapes. The mass of both kinds of solids is determined by using a balance. The volume of a regular solid can be determine by first measuring its dimensions and then calculating its volume using the geometric formulas in Figure 1. Most earth materials are irregular shaped solids whose volumes are determined by the amount of water they displace when submerged. Even careful measurements of density contain some error. The percent error is the amount by which any measurement differs from the accepted value. Percent error is determined by using the following formula. difference between measured and accepted value Percent error = x 100 accepted value MATERIALS balance cubic block metric ruler Area and Volume worksheet rectangular block cylindrical block 100-mL graduated cylinder 50-mL beaker wet limestone chips paper towels Procedures Part A: Finding the Density of Regular Solids Record all measurements and calculations in procedure steps 1-7 in the Density Chart for Regular solids (Table 1). 1. Use the balance to measure and record the mass of the cube to the nearest tenth of a gram. 2. Using the metric ruler, measure and record the dimensions of the cube. 3. Using the formula in Figure 1 or from the Area and Volume worksheet, compute and record the cube's volume. 4. Calculate the density of the cube by using its mass and volume. 5. Repeat procedure steps 1-4 using the rectangular block. Name __________________________________ Period _____ Date ____________ 6. Repeat procedure steps 1-4 using the cylinder. Record the radius of the cylinder in the column labeled width. 7. Obtain the accepted value for the density of each object from your teacher. Use this value to calculate the percent error for each object. Table 1: Density Chart for Regular Solids* Object Mass (g) Length (cm) cube Width (cm) Height (cm) -------- -------- Volume (mL) Density (g/cm3) Percent Error rectangle cylinder --------- *Round off all values to the nearest tenth of the given unit. Part B: Finding the Density of Irregular Solids Record all measurements and calculations from procedure steps 8-17 in the Density Chard for Irregular Solids (Table 2) 8. Pour about 50 mL water into a 100-mL graduated cylinder. Record the volume of the water in the cylinder to the nearest 0.1mL. Leave the water in the cylinder. 9. Use the balance to determine the mass of the cylinder plus the water. Record the mass. 10. Use the 50-mL beaker to obtain a sample of wet limestone chips. Select 5 chips and blot them with a paper towel. Slide the chips into the cylinder, without splashing any water. Record the volume of the water plus the chips. Leave the chips in the cylinder. 11. Subtract the volume you calculated in Step 8 from the volume in Step 10. Record this value as the volume of the 5 chips. 12. Use the balance to find the mass of the cylinder containing the water and the 5 chips. 13. Subtract the mass of the cylinder and water from the mass of the cylinder, water, and 5 chips. Record your result as the mass of the 5 chips. 14. Use the formula D = m/V to determine the density of the 5 limestone chips. Record the density in the table. 15. Take five more chips and blots them with at towel. Repeat procedures steps 10-14 using a total of 10 chips. Then repeat using totals of 15. Record each set of data. 16. Plot the data for the mass and volume on the chips on the graph in Figure 2. Draw a straight line on the graph that comes as close as possible to most of the points. Table 2: Density Chart for Irregular Solids* Solid Mass Cylinder + Water (g) Mass Cylinder + Water + Chips (g) Mass Chips (g) Volume Water (mL) 5 chips 10 chips 15 chips *Round off all values to the nearest tenth of the given unit. Volume Water + Chips (mL) Volume Chips (mL) Density Chips (mL) Name __________________________________ Period _____ Date ____________ Figure 2 Analysis and Conclusions 1. Why are the densities of the regular solids about the same? 2. If one block were cut in two pieces, what would be the density of each piece? Why? 3. Why do the points on the graph form a nearly straight line? What does that say about the ratio of mass to volume? (Hint: Note that the slope of your line equals the density of the chips.) 4. On the graph, draw and label a line for a material with a density of 1.0 g/mL (slope equals m/V or 1.0 g/mL). Where is this line relative to the line for the chips? Explain. 5. On the graph, draw and label a line for a material with a density of 4.0 g/mL. Where is this line relative to the line for the chips? Explain. 6. What are some potential sources of error in this investigation? Name __________________________________ Period _____ Date ____________ Name __________________________________ Period _____ Date ____________ Answer Key PURPOSE To gain experience with lab equipment and procedures, including making measurements, computing, and graphing. MATERIALS Provide students with a copy of Lab Sheet 1. A copy master is provided on page 137 of the teacher's laboratory manual. The cube, cylinder, and block should be of the same material. Limestone chips are sometimes called marble chips. Soak them before use. PROCEDURE 1., 6. You might want to copy the Density Charts for students in advance. 2., 3., 4. You may want to write the following equations on the board: density = mass volume volume of cube or rectangle: volume = length x width x height volume of cylinder with radius r: volume = π x radius2 x height 5. Accepted densities for objects can be given, measured by you, or determined by class consensus. 8. Soaking the chips in advance prevents distortion of the measurements by water absorption. Splashing water out of the cylinder may change the measurements. ANALYSIS AND CONCLUSIONS 1. The densities are about the same because all three objects are made of the same material. 2. The density of each piece would be the same as the density of the original piece because the material is still the same. 3. All the limestone chips are made of the same material with the same density, so they have the same ratio of mass to volume. Therefore, the graph is a straight line. 4. The line representing a material with a density of 1.0 g/mL is below (or to the right of) the limestone line because the material is less dense than the limestone chips. 5. The line representing a material with a density of 4.0 g/mL is above (or to the left of) the limestone line because the material is denser than the limestone chips. 6. Measuring the mass inaccurately, rounding off numbers, excess water remaining on the chips, splashing water out of the cylinder Refer students to Safety in the Earth Science Laboratory on pages xxii-xxiii.