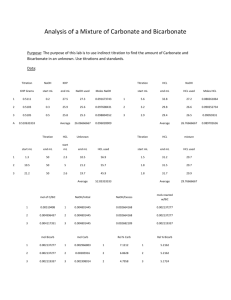
Review questions
advertisement

CHAPTER 23 THE EQUILIBRIUM EMPIRE STRIKES AGAIN Name: QUESTIONS 23.1 Acids, bases and water 1. 2. (a) Give an operational definition of metal. (b) Give a conceptual definition of metal. (a) What are the conjugate acid–base pairs in the following reaction? H2SO4 + HOH → H3O+ + HSO4– (b) What is the conjugate acid of Br–? (c) What is the conjugate base of HNO3? 23.2 Testing solutions of acids and bases 3. (a) What is the pH of a solution with a H+ concentration of: (b) 4. (i) 1 × 10–4M (ii) 1 × 10–8M (iii) 0.02M? Identify the solutions in part (a) as acids or bases. Determine the pOH and the pH of the following solutions, each at 25 °C, which have OH– concentrations of: (a) 0.01M (b) 1.0M (c) 10–3M. © John Wiley & Sons Australia, Ltd 1 QUEENSLAND CHEMISTRY 5. 6. How does the pOH change with (a) increasing [H+] (b) increasing [OH–]? A vinegar solution has a pH of 3.0. Assuming the temperature is 25 °C, determine its: (a) H+ concentration (b) OH– concentration (c) pOH. 23.3 Acid/base calculations 7. (a) What is the pH of a 10 mL sample of 0.02M HNO3? (b) What amount of H+ is present in the sample of the acid? 8. Ka of CH3COOH is 1.8 × 10–5 at 25 °C. What is the pH of a 0.10M CH3COOH solution? 9. Calculate the pH and pOH of a 20 mL sample of 0.20M KOH. 10. What would be the acetate concentration of a solution of 1.0M CH3COOH? Ka of CH3COOH is 1.8 × 10–5. 11. What is the acetate ion concentration of a solution that is 1M in CH3COOH and 1M in HCl? 12. Suppose 20 mL of 0.20M acetic acid had been mixed with 40 mL of 0.14M sodium hydroxide. Calculate the pH of the resulting solution. 23.4 Maintaining a desired pH 13. Calculate the pH if 0.01 mole of NaOH had been added with no change in volume. 23.5 Titrations revisited 14. Sketch a curve showing the pH changes in a titration in which a strong acid is added to a solution of a strong base. 15. Compare the graphs of the acetic acid – sodium hydroxide titration, the hydrochloric acid – ammonia titration and the hydrochloric acid – sodium hydroxide titration. What are the © John Wiley & Sons Australia, Ltd 2 QUEENSLAND CHEMISTRY similarities? What are the differences? 16. Explain why methyl orange or methyl red could be used as an indicator in the ammonia titration but phenolphthalein and bromthymol blue could not. 17. A solution consists of equi-molar mixtures of a weak acid and a weak base. Indicate whether the following solutions would be acidic, neutral or basic. (a) The acid is weaker. (b) The base is weaker. (c) The acid and base are equally weak. Review questions Note: unless stated otherwise, assume a temperature of 25 °C. 1. 2. Distinguish between the following pairs of terms. (a) Lewis acid and Lowry–Brønsted acid (b) Conceptual definition and operational definition Identify each of the following as a strong/weak acid/base and then write equations for the reactions of each with water. (a) HI (b) NH3 (c) H2SO3 (d) HNO3 (e) H2SO4 (f) CH3NH2 (g) H3PO4 (h) H2S (i) CH3CH2COOH © John Wiley & Sons Australia, Ltd 3 QUEENSLAND CHEMISTRY (j) 3. 4. 5. H2CO3 Identify the conjugate acid of each of the following. (a) NH3 (b) H2O (c) Br– (d) HS– (e) SO42– (f) S2– Identify the conjugate base of each of the following. (a) H2O (b) HS– (c) HCl (d) HPO32– (e) NH4+ (f) HSO3– Complete each of the following equations and indicate the conjugate acid–base pairs in each. (a) NH3 + HCl → (b) H2SO4 + H2O → (c) CH3COOH + NaOH → (d) H2CO3 + HS– → (e) HS– + OH– → (f) HS– + H3O+ → © John Wiley & Sons Australia, Ltd 4 QUEENSLAND CHEMISTRY 6. 7. 8. 9. Calculate the pH of solutions in which the [H+] is: (a) 10–3 (b) 0.04 (c) 1.6 × 10–6 (d) 10 (e) 8.5 × 10–12 Calculate [H+] and [OH–] of solutions that have a pH of: (a) 3 (b) 10.2 (c) 7. Calculate the pOH of solutions with hydroxide concentrations of: (a) 0.015M (b) 4.0 mol L–1 (c) 10M (d) 1.25 moles in 100 mL. Calculate the pH and the pOH of the following solutions. (a) 100 mL of 1.45M HCl (b) 5.0 mL of 0.001M Ca(OH)2. Assume complete ionisation. (c) 8.0 g of NaOH dissolved in 400 mL of solution (d) 0.15 mol of HCl gas dissolved in 100 mL of solution 10. 4.65 g of KOH are dissolved in water to make 250 mL of solution. (a) List all of the entities found in the solution. (b) Determine the concentration of: © John Wiley & Sons Australia, Ltd 5 QUEENSLAND CHEMISTRY (c) (i) OH– (ii) H+. Determine the pH of the solution. 11. 5.0 mL of 16M HNO3 are diluted to a total volume of 300 mL with distilled water. For the newly prepared solution, calculate: (a) the molarity (b) the hydrogen ion concentration (c) the pH (d) the mass of NaOH that must be added to increase the pH of the nitric acid solution to 2. 12. Is it possible to use the materials indicated to prepare the following solutions? Concentrated HCl, HNO3 and H2SO4 solutions are 12M, 16M and 18M respectively. The solubility of KOH is 126 g per 100 mL of water. (a) An aqueous solution with a pH of –1 using nitric acid (b) An aqueous solution with a pH of –1.1 using hydrochloric acid (c) An aqueous solution with a pH of –2 using any of the acids available (d) An aqueous solution with a pH of 14 using KOH (e) An aqueous solution with a pH of 15 using KOH 13. Calculate the pH of a solution formed by mixing: (a) 10 mL of 0.15M HCl and 12 mL of 0.18M NaOH (b) equal volumes of 0.200M HCl and 0.400M NaOH (c) equal volumes of 0.100M H2SO4 and 0.200M KOH (d) 25 mL of 0.865M HCl and 30 mL of 0.752M KOH (e) 20.0 mL of 0.336M H2SO4 and 38.0 mL of 0.340M NaOH. © John Wiley & Sons Australia, Ltd 6 QUEENSLAND CHEMISTRY 14. Acetic acid has an ionisation constant of 1.8 × 10–5 at 25 °C. What is the pH of a 1.5M solution of acetic acid? 15. A 0.1M benzoic acid solution has a pH of 2.6. What is Ka of the acid? 16. What is the pH of a solution of 0.01M HClO? Ka = 2.95 × 10–8. 17. What is the pH of a 0.24M solution of ammonia? Kb= 1.8 × 10–5. 18. A 0.010M solution of a monoprotic organic acid is 1.8% ionised. (a) What is the ionisation constant of the acid? (b) What is the pH of the solution? 19. Calculate the pH of a solution made by mixing 10 mL of 1.0M CH3COOH and 10 mL of 1.0M NaCH3COO solution. Ka for CH3COOH is 1.8 × 10–5. 20. A solution contains equal volumes of 0.12M NH3 and 0.85M NH4Cl. What is the pH of the solution? Kb for NH3 is 1.8 × 10–5. 21. Sufficient 1.0M HCl has been added to a 0.1M solution of H2S to adjust the pH to 2. K1 and K2 for the successive ionisations of H2S are 9.1 × 10–8 and 1.1 × 10–15. What is [S2–] in the final solution? 22. The ionisation constant for water at 0 °C is 1.14 × 10–15. At this temperature: (a) what is the hydrogen ion concentration in water (b) what is the pH of water (c) what is the hydrogen ion concentration at a pH of 7 (d) what is the pH when the hydroxide concentration is 10 × 10–7M? 23. At 25 °C, Kw = 1.0 × 10–14. At 60 °C, Kw = 9.4 × 10–14. (a) Is the ionisation of water an endothermic or exothermic reaction? Explain. (b) If the pH of a solution at 60 °C is equal to 6, what is its pOH? 24. Which of the following could be used to make buffer systems? Explain your reasoning. (a) HCl and CH3COOH © John Wiley & Sons Australia, Ltd 7 QUEENSLAND CHEMISTRY (b) NaCl and HCl (c) NaOCl and HOCl (d) H2CO3 and NaHCO3 (e) NH3 and NH4NO3 (f) NH3 and HF 25. (a) (b) Calculate the pH of a benzoic acid – sodium benzoate buffer system containing 0.1M C6H5COOH and 0.1M C6H5COONa. Ka of benzoic acid is 6.5 × 10–5. Calculate the pH of the system if 0.0010 mole of gaseous HCl is added to 100 mL of the buffer solution. 26. A buffer is prepared by dissolving 20 g of acetic acid and 20 g of sodium acetate in water and diluting the solution to a total volume of 100 mL. Ka of the acid is 1.8 × 10–5. What is the pH of the buffer? 27. A buffer solution is prepared by mixing solutions of a weak monoprotic acid (HA) with a salt of the acid (MA). Ka of the acid is 1.5 × 10–6, and the concentrations of both the acid and the salt are 0.10M. (a) What is the concentration of H+ in the buffer solution? (b) What is the pH of the buffer solution? (c) Explain what would happen in solution with the addition of a small amount of: (i) HCl (ii) NaOH. 28. A buffer is prepared by mixing 100 mL of 0.20M NH3 with an equal volume of 0.20M NH4Cl. Kb for ammonia is 1.8 × 10–5. (a) What is the pH of the buffer solution? (b) What is the pH of the solution after the addition of 10.0 mg of gaseous HCl to 50 mL of buffer? Assume no change in volume. (c) What is the pH of the solution after the addition of 0.010 mol of gaseous HCl to another 50 mL of buffer? Assume no change in volume. © John Wiley & Sons Australia, Ltd 8 QUEENSLAND CHEMISTRY (d) What is the pH of the solution after the addition of 0.010 mol of solid NaOH to the remaining 100 mL of buffer? Assume no change in volume. 29. Explain: (a) the purpose of an indicator in an acid–base titration (b) how acid–base indicators work (c) why only small amounts of indicator are used. 30. Compare the curves of pH versus volume of titrant: (a) when a strong base is added to a strong acid and when a strong acid is added to a strong base (b) when a strong base is added to a weak acid and when a weak base is added to a strong acid. 31. A 25.00 mL sample of acetic acid is titrated with 0.1035M NaOH using phenolphthalein as an indicator. 30.26 mL of base are needed to reach the endpoint. What is the concentration of the acid? 32. 0.5000 g of anhydrous analytical reagent grade Na2CO3 are dissolved in distilled water and made up to 100 mL in a volumetric flask. When a 30.0 mL aliquot of the standard solution is titrated with an HCl solution, 28.62 mL of the acid are needed to reach the endpoint. What is the concentration of the hydrochloric acid? 33. Palmitic acid, an ingredient in palm oil, is used in making soap. C15H31COOH + NaOH → C15H31COO– Na+ + HOH Palmitic acid Soap How many millilitres of 0.9845M NaOH are needed to react completely with 5.000 g of the acid? 34. A sample of rainwater collected near a copper smelter is analysed for its acid content. Titration of a 100 mL sample of the rainwater requires a titre of 22.40 mL of 0.01225M NaOH. Assuming that the acid present in rainwater is primarily H2SO3 and that the indicator used responds to the second ionisation of the acid, what is the concentration of H2SO3 in the rainwater? 35. Lactic acid (C2H5OCOOH) is the sour ingredient in sour milk and in buttermilk. A recipe that calls for sour milk or buttermilk usually also calls for bicarbonate of soda (NaHCO3). Calculate the mass of NaHCO3 needed to react completely with 250 mL (1 cup) of 0.0510M lactic acid. © John Wiley & Sons Australia, Ltd 9 QUEENSLAND CHEMISTRY Notes: © John Wiley & Sons Australia, Ltd 10