POLA_24806_sm_suppinformation
advertisement

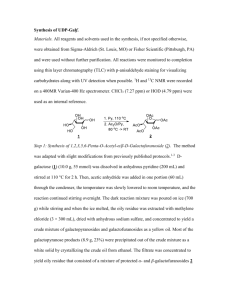
Supporting Information for Synthesis and structure-activity relationship of several aromatic ketone-based two-photon initiators Zhiquan Li, Marton Siklos, Niklas Pucher, Klaus Cicha, Aliasghar Ajami, Wolfgang Husinsky, Arnulf Rosspeintner, Eric Vauthey, Georg Gescheidt, Jürgen Stampfl, Robert Liska e-mail: robert.liska@tuwien.ac.at 1. Syntheses of dihalide aromatic ketones 2. Processing Windows of TPA PIs 3. Photodegradation effect (z-scan analysis) 4. References 1. Syntheses of dihalide aromatic ketones Bis(4-iodophenyl)methanone (4a)1 11.85 g Diphenylmethane (70.44 mmol) and 17.90 g molecular iodine (70.53 mmol) were dissolved in 70 mL glacial acetic acid in a round bottom flask. The solution was stirred at 40 °C and a mixture of 6.5 mL nitric acid (63%) and 15.0 mL concentrated sulfuric acid (98%) was added dropwise. Then the reaction mixture was heated under reflux and 37 mL nitric acid (50%) was added. The solution was heated until brown gas formation stopped. The mixture was cooled and the precipitate was filtered off, washed with water until neutral washings, dried in vacuum, and recrystallized from dioxane to give 21.4g white needle-shape crystal. Yield: 70%. MP: 233-235 °C. Lit: 234-235 °C. 1H NMR (200 MHz, CDCl3) δ 7.84 (d, J = 8.2 Hz, 4H), 7.47 (d, J = 8.3 Hz, 4H). 13C NMR (50 MHz, CDCl3) δ 195.55, 137.73, 136.36, 131.30, 100.46. 2,7-Diiodo-9H-fluoren-9-one (4b)2 A solution of 7.51 g (41.62 mmol) of fluorenone and 10.62 g (41.84 mmol) of molecular iodine in a mixture of 100 mL of glacial acetic acid and 3 mL of chloroform (to wash off sublimed iodine) was heated under reflux. Subsequently, a mixture of 2 mL of concentrated sulfuric acid and 4 mL fuming nitric acid was added dropwise, and the mixture was heated until brown gas formation stopped. The reaction mixture was cooled, and the precipitate was filtered off, washed with water until neutral washings, dried in vacuum, and recrystallized from ethyl acetate to give 13.29 g yellow needles. Yield: 73%. MP: 202-204 °C. Lit: 205-206 °C. 1H NMR (200 MHz, Acetone) δ 8.01 (dd, J = 7.8, 1.5 Hz, 2H), 7.92 (d, J = 1.4 Hz, 2H), 7.64 (d, J = 7.9 Hz, 2H). 13 C NMR (50 MHz, CDCl3) δ 190.93, 143.41, 142.92, 134.79, 133.51, 122.11, 94.46. 3,6-Dibromophenanthrene-9,10-dione3 A mixture of 7.03 g (33.4 mmol) of 9,10-phenanthrenequinone and 0.35 g (mmol) of dibenzoyl peroxide in 50 mL of nitrobenzene was irradiated with a 100 W tungsten light bulb at a distance of 10 cm. Over the course of 0.5 h, 5.0 mL of molecular bromine (97.6 mmol) was dripped in. The starting material slowly dissolved and soon precipitation of product started. Irradiation and stirring were continued for a further 5 h. The reaction mixture was kept at room temperature overnight, suspended in 50 mL of Et2O, filtered and thoroughly washed with Et2O. The dibrominated compound was obtained as 6.41 g of ocher yellow crystals. Yield: 52%. Mp: 284-287 °C. Lit: 285286 °C. 3,6-Dibromo-9H-fluoren-9-one (4c)4 A warm saturated potassium hydroxide solution was prepared by dissolving 90 g KOH in 35 mL of water. Then 19.30 g of crude 3,6-dibromophenanthrene-9,10-dione (52.73 mmol) were added and the thick suspension was heated under reflux for 1 h. Over the course of 2.5 h, the mixture was refluxed and treated with 47 g KMnO4 in small portions. A supplemental addition of 20 mL of water was required to enable continued stirring of the reaction mixture. After an additional 2 h of refluxing, the reaction was cooled overnight, cautiously acidified with 30% H2SO4 (evolution of CO2) and the remaining oxidizing agent reduced with NaHSO3 solution. The beige precipitate was filtered, washed thoroughly with water then with acetone. After air drying, the substance was purified by recrystallization from DMSO, removing the solvent adhering to the surface of the crystals after filtration with acetone then CHCl 3. The product was obtained as 10.05 g of light yellow crystals. Yield: 56%. Mp: 320323 °C. Lit:5 323-324 °C. 1H NMR (CDCl3): δ 7.71 (d, J=1.5 Hz, 2H), 7.58 (d, J=7.9 Hz, 2H), 7.52 (dd, J=1.4 Hz, J=7.8 Hz, 2H). 2,6-Diiodo-9,10-anthraquinone (4d)6 10.02 g of 2,6-diaminoanthraquinone (42.00mmol) were dissolved in 20 mL acetonitrile and subsequently 11 mL concentrated sulfuric acid was added. The resulting slurry was cooled to 0 °C in an ice bath and then a precooled solution (0 °C) of sodium nitrite (5.81 g, 84.11mmol) in water (15 mL) was slowly added. The reaction mixture was then stirred for 30 min at 0 °C and then added to a round bottom flask containing a solution of potassium iodide (20.01 g, 120.51 mmol) in water (50 mL) cooled to 0 °C. Then the solution was allowed to warm to room temperature with stirring for 1 h. The product was then recovered by filtration. The filtrate was washed with water until neutral washings, dried in vacuum, and recrystallized from toluene to give 11.59 g light brown crystalline solid. Yield: 60%. Mp: 282-284 °C. 1H NMR (200 MHz, CDCl3) δ 8.62 (d, J = 1.7 Hz, 2H), 8.15 (dd, J = 8.2, 1.8 Hz, 2H), 7.96 (d, J = 8.2 Hz, 2H). 13C NMR (50 MHz, CDCl3) δ 181.57, 143.36, 136.33, 132.07, 128.73, 102.70. 2. Processing Windows of TPA PIs Processing Windows of PIs with different feed rate and laser power. (a) R1; (b) B3K; (c) B3BP; (d) BB3AN; (e) 3,6-B3FL; (f) B3FL; (g) BB3FL. 3. Photodegradation effect (z-scan analysis) Photodegradation effect of BB3FL at different volumetric flow rates (0 and 4 mL/h) 4. References 1. Novikov, A. N.; Slyusarchuk, V. T.; Chaikovskaya, L. G.; (Tomsk Polytechnic Institute, USSR; Tomsk Medical Institute). Application: SU 642288 A1 19790115, 1979. 2. Abashev, G. G.; Lebedev, K. Y.; Osorgina, I. V.; Shklyaeva, E. V. Russ. J. Org. Chem 2006, 42, 1873-1876. 3. Bhatt, M. V. Tetrahedron 1964, 20, 803-821. 4. Ipaktschi, J.; Hosseinzadeh, R.; Schlaf, P.; Dreiseidler, E.; Goddard, R. Helv. Chim. Acta 1998, 81, 1821-1834. 5. Barker, A.; Barker, C. C. J. Chem. Soc 1954, 870-873. 6. Gouloumis, A.; Gonzalez-Rodriguez, D.; Vazquez, P.; Torres, T.; Liu, S.; Echegoyen, L.; Ramey, J.; Hug Gordon, L.; Guldi Dirk, M. J. Am. Chem. Soc 2006, 128, 12674-12684.