reagent preparation
advertisement
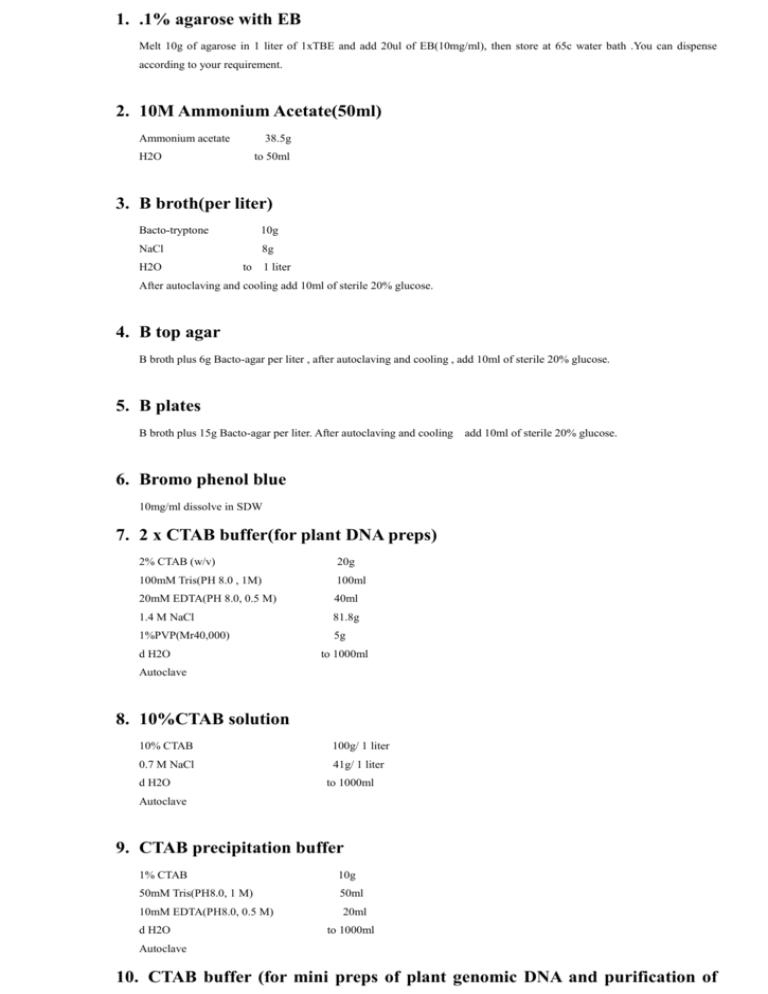
1. .1% agarose with EB Melt 10g of agarose in 1 liter of 1xTBE and add 20ul of EB(10mg/ml), then store at 65c water bath .You can dispense according to your requirement. 2. 10M Ammonium Acetate(50ml) Ammonium acetate 38.5g H2O to 50ml 3. B broth(per liter) Bacto-tryptone 10g NaCl 8g H2O to 1 liter After autoclaving and cooling add 10ml of sterile 20% glucose. 4. B top agar B broth plus 6g Bacto-agar per liter , after autoclaving and cooling , add 10ml of sterile 20% glucose. 5. B plates B broth plus 15g Bacto-agar per liter. After autoclaving and cooling add 10ml of sterile 20% glucose. 6. Bromo phenol blue 10mg/ml dissolve in SDW 7. 2 x CTAB buffer(for plant DNA preps) 2% CTAB (w/v) 20g 100mM Tris(PH 8.0 , 1M) 100ml 20mM EDTA(PH 8.0, 0.5 M) 40ml 1.4 M NaCl 81.8g 1%PVP(Mr40,000) 5g d H2O to 1000ml Autoclave 8. 10%CTAB solution 10% CTAB 100g/ 1 liter 0.7 M NaCl 41g/ 1 liter d H2O to 1000ml Autoclave 9. CTAB precipitation buffer 1% CTAB 10g 50mM Tris(PH8.0, 1 M) 50ml 10mM EDTA(PH8.0, 0.5 M) 20ml d H2O to 1000ml Autoclave 10. CTAB buffer (for mini preps of plant genomic DNA and purification of large scale genomic DNA) 0.2M Tris-HCl(PH7.5, 1M) 200ml 0.05M EDTA(PH8.0, 0.5M) 100ml 2.0M NaCl 116.7g 2%W/V CTAB 20g d H2O to 1000ml Autoclave 11. CTAB solution(for antirrhinum DNA preps) 50mM Tris PH 7.45(12.5ml 1M Tris) 10mM EDTA PH8.0(5ml 0.5M EDTA) 2% CTAB(5.0 g) H2O to 250ml 12. 50 x Denhardt’s 10 g ficoll(Type 400) 10 g polyvingpyrrolidone(MW.360,000) 10 g B S A(Fraction v) H2O to 1000ml 13.DNA miniprep extraction buffer(Antirrhinum leaves) 0.5M EDTA (PH8.0) 10ml(final 50mM) 5MNaCl 2 ml (final 0.1M) 1 MTris(PH8.0) 10ml(final 0.1M) 10%SDS 10ml(final 1%) H2O to 100ml 14.1M Dithiothnetol(DTT) 10ml DTT 1.55g 10mM sodium acetate(PH 5.2) to 10ml Store at –20c 15.0.5M EDTA (PH8.0) EDTA 2H2O 186.1g H2O 800ml Adjust PH to 8.0 with NaOH(about 20g of NaOH was needed) and fix the volume to 1000ml, autoclave. 16.10mg/ml Ethidium Bromide(20ml) Ethidium bromide H2O 0.2g to 20ml Mix well, store at 4c in dark. Handle with extreme caution 17.Formaldehyde gel(100ml) Agrose 1-1.5g 10 x MOPS buffer 10ml water 73ml Dissolve agrose and cool to 50c. Add 17ml of formaldehyde (37%v/v sulotion). Mix and pour immediately. Running buffer is 1x MOPs buffer. 18.X-gal 20mg/ml in dimethylformamide(DMF) use a glass or polypropylene tube,wrap in aluminum foil and store at –20c. 19.Hybridization solution( for southern and northern) 1 liter 20x SSC 300ml( final 6x SSC) 50x Denhandt’s 100ml( final 5x Denhardt’s) 10% SDS 50ml( final 0.5%) SDW to 1000ml 10mg/ml of ssDNA added to the final 20ug/ml 20.IPTG 2g of IPTG dissolve in 100ml of H2O, Sterilize by filtration, store at –20c at 1ml aliquots. 21.5M KAc(pH~4.8) 461g KAc in H2O, adjust PH with glacial acetic acid and autoclave. 22. LB medium-1 liter bacto-trypton 10g bacto yeast extract 5g NaCl 10g H2O 950ml Adjust PH to 7.0 with 5N NaOH and fix the volume to 1000ml, autoclave. For LB plates, add 15g of bacto-agar to 1 liter medium, autoclave; For LB top agar, add 7g of bacto-agar per liter LB medium. 23.RNA gel loading buffer(100ml) Glycerol 50ml Bromophenol blue 10mg SDW to 100ml 24.1M MgSO4 MgSO4 7H2O 246.47g + H2O to 1000ml 25.10 x MOPS MOPS 46.2g (final 0.2M) EDTA 3.72g (final 0.01M) NaAc 41g (final 0.5M) SDW 800ml Adjust to 7.0 with HAc and fix the volume to 1000ml with SDW. 26.1M MgCL2 MgCL2 6H2O 20.3g + H2O to 100ml 26.10 x phosphate buffer saline (PBS) NaCL 80g KCL 2g Na2HPO4 7H2O 26.8g KH2PO4 2.4g H2O 800ml Adjust the PH to 7.4 with HCL and fix the volume to 1000ml with H2O. 27.10 x PCR buffer(10ml) 1M Tris-HCL(PH 8.3) 1ml (final 100mM) 1M KCL 5ml (final 500mM) 1M MgCL2 0.2ml (final 20mM) 1% Gelatin 1ml (final 0.1% w/v) SDW to 10ml Make the solution to 10ml by adding SDW(no need for autoclaving) 28.RNase stock solution 10mg/ml of RNase A in H2O. Heat at boiling(in a water bath) for at least 10 min to destroy any DNase and then kept frozen until needed. 29.3M Sodium acetate 408.1g of NaAc 3H2O in 800ml of H2O, adjust the PH to 5.2 with glacial acetic acid, fix the volume to 1 liter with H2O. Autoclave. 30.20 x SSC NaCl 175.3 g Sodium citrate 88.2 g H2O 800 ml PH to 7.0 with a few drops of 10N of NaOH. Fix the volume to 1 liter and autoclave. 31.5M NaCl 292.2 g of NaCl dissolve in 800ml of H2O and fix the volume to 1000ml, autoclave. 32.10% SDS 100g of SDS in 900ml of H20, heat the solution to 68c to helping dissolve, adjust the PH to 7.2 by adding a few drops of concentrated HCl(there is no need to sterilize). 33.SOB medium per liter Trypton 20g Yeast extract 5g NaCl 0.5g 250mM KCl 10ml Add water to 900ml. Adjust PH to 7.0 and add water to 990ml. Autoclave, cool to room temperature and add 10ml of sterile solution of 1M MgCl2 before use. 34.SOC medium per liter SOB with the addition of 20ml filter sterilized 1M glucose per liter of SOB. 35.1M Tris 121.1 g Tris BASE H2O 800ML Adjust the PH with HCl and fix the volume to 1000ml. Autoclave. 36.1 x TE Tris-HCl (PH 8.0) 10mM EDTA (PH 8.0) 1mM 37.50 x TAE Buffer/liter Tris base 242g Glacial acetic acid 57.1ml 0.5M EDTA,PH8.0 100ml H2O to 1 liter Adjust PH to 8.5. 38.10x TBE buffer/liter Tris base 108 g Boric acid 55 g 0.5M EDTA,PH8.0 40ml H2O to 1 liter 39.2x YT bacto-tryptone 16 g bacto-yeast extract 10 g NaCl 5g Adjust PH to 7.0 with 5N of NaOH and fix the volume to 1 liter. Autoclave.