Potato Battery
advertisement

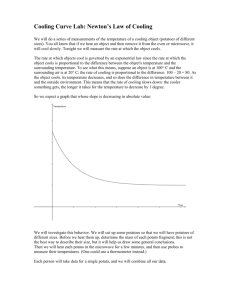
Potato Battery Grade Level: 6 Strand: Understanding Matter and Energy Topic: Electricity and Electrical Devices Lesson Expectation: Design, build, and test a device that produces electricity (e.g., a battery built from a lemon or potato; a wind turbine) Required Materials: - 3 potatoes - 4-6 pennies (copper pennies) - 4-6 galvanized nails (or zinc nails) - 8 alligator clips - Copper wires - Low-voltage calculator or low-voltage light bulb Procedure: 1. Insert a galvanized nail into each potato 2. Insert a penny into each potato. Be sure to keep the penny away from the nail (not touching) 3. Wrap the ends of a copper wire around an alligator clip (2 alligator clips per one piece of copper wire). Do this until all the alligator clips are connected to copper wire. 4. Connect an alligator clip to a galvanized nail in potato 1; attach the other alligator clip to a penny in potato 2. Then connect the alligator clip to the galvanized nail in potato 2 and attach the other end of the alligator clip to the penny in potato 3. 5. All the potatoes are connected by wires. Connect an alligator clip to the galvanized nail in potato 3 and connect the other end to the negative end of the calculator. 6. Attach the last set of alligator clips to the nail in potato 1 and to the positive end of the calculator 7. With the circuit complete the calculator should turn on Scientific Explanation: A potato contains chemical energy. An electrochemical cell converts chemical energy into electrical energy. A type of electrochemical cell is the potato battery. This is due to the transfer of electrons between the zinc nail and copper penny. The potato transfers the electrons (conducts electricity), but keeps the zinc ions and copper ions separate. This separation forces the electrons to transfer over the copper wires (generate current). This then channels the energy into the clock. If the zinc ion and copper ions touched they would react but would only generate heat. The potato battery does not contain enough power to shock a person, but can power small electronics. Other considerations: Other foods can also act as an electrochemical cell. Try using citrus fruits, pickles, or cola. Definitions: Electricity – energy created by moving charged particles (such as electrons, positrons, and ions) Electrons – elementary particle; a stable negatively charged elementary particle with a fundamental constituent of matter and orbits the nucleus of an atom Chemical energy – the energy released or absorbed during the decomposition or formation of compounds Electrical energy - is the presence and flow of an electric charge Electrochemical cell - is a device capable of either deriving electrical energy from chemical reactions Sources: http://www.kidzworld.com/article/4726-how-potato-batteries-work http://chemistry.about.com/od/chemistryhowtoguide/a/fruitbattery.htm http://www.youtube.com/watch?v=AY9qcDCFeVI http://www.youtube.com/watch?v=ufoOJfzro2c By: George Franko & Meagan Warnock