Measurement of Kla in a Sparged Bioreactor
advertisement

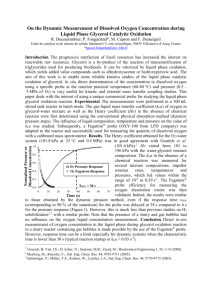
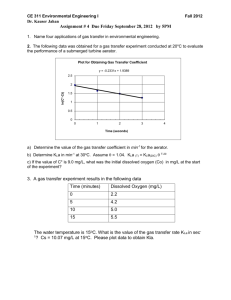
Measurement of Kla in a Sparged Bioreactor Introduction Dissolved oxygen is an important substrate in aerobic fermentations. Since oxygen is sparingly soluble in water, it may be the growth-limiting substrate in these fermentations. For bacteria and yeast cultures, the critical oxygen concentration is about 10% to 50% of the saturated DO (dissolved oxygen concentration). Above this critical concentration, the oxygen concentration no longer limits growth. For optimum growth it is therefore important to maintain the DO above this critical level by sparging (bubbling gas through) the fermentor with air or pure oxygen. Of course, to be effective, the mass transfer rate from the gas bubbles to the liquid broth must equal or exceed the rate at which growing cells take up the oxygen. Oxygen transfer is usually limited by the liquid film surrounding the gas bubbles. The rate of transport is given by: N O2 k L a C * C L (1) where kL is the oxygen transport coefficient (cm/h), a is the gas-liquid interfacial area (cm2/cm3), kLa the volumetric oxygen transfer coefficient (h-1), C* is saturated DO concentration (mg/l) (approx. 7 mg/l at 25 deg. C and 1 atm.), CL is the actual DO concentration in the liquid (mg/l), and N O2 is the rate of oxygen transfer (mg O2/ l h) (Shuler and Kargi, 1992) In this lab you will use a “gassing-in” method to measure kLa. Linek, Sinkul, and Benes describe this method in several excellent review articles. The fermentor should be filled approximately 2/3 full with water. Valves on the back of the fermentor allows you to first de-gas the liquid by sparging with nitrogen, and then introduce a step change to pure oxygen. The rate of gas transfer to the liquid is determined by using a DO probe that is mounted in the fermentor head. This probe is connected to a DO meter, which in turn is connected to a data acquisition (DAQ) system on the PC. Note that the DO probe we are using has a relatively slow response time, and you may want to take probe dynamics into account. The value of kLa may be determined by analysis o f the concentration vs. time data. The DAQ system is also set up to automatically monitor impeller (stirrer) speed and fermentor temperature, and to store it in an Excel spreadsheet. You will want to check the calibration on these parameters to make sure you are getting accurate data. Assignment 1) Design and perform an experiment to measure probe response time. 2) Design and perform an experiment to characterize the dependence of k La on impeller speed and on superficial gas velocity. Use at least a 24 design = 16 experiments. The gas flow rate should be a minimum of 1.0 L/min and a maximum of 14.0 L/min. The stir rate should be a minimum of 300 RPM and a maximum of 900 RPM. Use the results of items 1-3 to determine coefficients/exponents a, b, c in an equation of the form: k L a ae b v sc (1) where e is power dissipated in volume of liquid phase (W/m3) and s is the superficial gas velocity in meters per second (note, you will need to measure the appropriate variables to obtain power input and superficial velocity). Compare to literature values, etc. 5) Use the “Historical Data” option under “2-Factorial Experiments” in the Design Expert software to analyze the data in terms of volumetric flowrate Q and stirring speed, N. Is there a significant 2-factor interaction? How is this interaction captured in eqn. 1? 6) Use the Brown article as a guide to discussing simplifications, modeling of the data. Explore any other areas of interest to you, other forms of correlations, etc. Starting References: Linek, V, Sinkule, J, and Benes, P, 1991, Critical Assessment of Gassing-In Methods for measuring kLa in Fermentors, Biotechnology and Bioenginering 38, 323-330 Linek, V and Benes, P, 1987, Critical Review and Experimental Verification of the Correct Use of the Dynamic Method for the Determination of Oxygen Transfer in Aerated Agitated Vessels to Water, Electrolyte Solutions and Viscous Liquids, Chemical Engineering J. 34, 11-34 Perry’s Handbook, 50th ed., section 28 Shuler, ML and Kargi, F., 1987, Bioprocess Engineering: Basic Concepts, Prentice Hall, NJ *Also any other good biochemical engineering text (i.e. Bailey and Ollis) , Perry’s Handbook (Liquid-Gas Systems, Biochemical Engineering), and misc. journal articles.