Counterpoint: Should Systemic Lytic Therapy Be Used for
advertisement

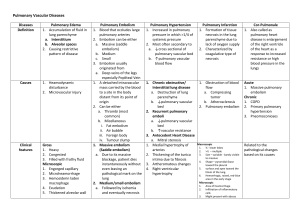
Counterpoint: Should Systemic Lytic Therapy Be Used for Submassive Pulmonary Embolism? No Chest - Volume 143, Issue 2 (February 2013) - Copyright © 2013 The American College of Chest Physicians - About This Journal Add Journals Issue Alert MDC Extra Article: This additional article is not currently cited in MEDLINE®, but was found in MD Consult's full-text literature database. Point/Counterpoint Editorials Counterpoint: Should Systemic Lytic Therapy Be Used for Submassive Pulmonary Embolism? No Kathryn L. Bilello, MD, FCCPa Susan Murin, MD, FCCPb,c,* a Department of Medicine, University of California San Francisco-Fresno Program, Fresno, CA b Department of Medicine, Division of Pulmonary, Critical Care and Sleep Medicine, University of California, Davis, School of Medicine, Sacramento, CA c Veterans Affairs Northern California Health Care System, Sacramento, CA * Correspondence to: Susan Murin, MD, FCCP, Division of Pulmonary, Critical Care and Sleep Medicine, University of California, Davis, School of Medicine, 4150 V St, Ste 3400, Sacramento, CA 95817 E-mail address: susan.murin@ucdmc.ucdavis.edu Financial/nonfinancial disclosures: The authors have reported to CHEST that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.Reproduction of this article is prohibited without written permission from the American College of Chest Physicians. See online for more details. PII S0012-3692(13)60073-2 DOI 10.1378/chest.12-2449 The outcome of acute pulmonary embolism (PE) depends on both the severity of the PE (clot burden) and the presence and severity of preexisting cardiopulmonary disease in the patient. Patients who develop shock or hypotension related to acute PE have a higher mortality than do patients with PE who are hemodynamically stable. Mortality from acute PE ranges from 70% in patients with cardiopulmonary arrest, to 30% in patients with cardiogenic shock, to 15% in patients with hypotension. [1] , [2] , [3] Consensus guidelines recommend treatment with thrombolysis, if not contraindicated, in hemodynamically unstable patients based on their high mortality rate and the physiologic rationale that they should benefit from the more rapid dissolution of the clot and resultant relief of the vascular obstruction that is known to occur with administration of lytic agents. [4] , [5] In contrast, patients without hypotension have a mortality ranging from 0% to 10%, [1] , [6] and guidelines recommend that they be treated with anticoagulation alone. [4] , [5] Within the group of patients with hemodynamically stable PE is a subgroup characterized as having submassive PE, variably defined on the basis of right ventricular (RV) enlargement, dysfunction, ischemia, or strain, as assessed by echocardiography, CT scan, serum markers (troponin or brain natriuretic peptide [BNP] levels), ECG criteria, or other means. [7] , [8] , [9] , [10] , [11] Multiple studies, including large registries, have reported a two to 2.5-fold increased risk of mortality in patients with normal BP and RV dysfunction compared with those without RV dysfunction. [1] , [3] , [8] , [12] Based on this elevated risk, some experts have advocated that patients with submassive PE be treated more aggressively and have recommended the use of thrombolytic therapy. [13] , [14] However, although patients with hemodynamically stable PE and RV dysfunction and/or ischemia may have a worse prognosis, not a single study has been published that demonstrates that normotensive patients with PE-induced RV dysfunction have a lower mortality when treated with thrombolysis. Furthermore, although carefully selected patients with submassive PE (deemed to be at low risk of bleeding) may benefit from thrombolysis, there is no validated prediction rule that allows us to select these patients up front. Thus, any blanket recommendation to treat all patients with submassive PE with thrombolysis cannot be supported. Studies have generally defined submassive PE on the basis of RV dysfunction, which has, itself, been defined in a wide variety of ways. Depending on the definition used, RV dysfunction can be identified in 27% to 55% of normotensive patients with PE. [7] , [15] , [16] Many, but not all, studies have identified higher short-term mortality in this population. [6] , [8] , [9] , [10] , [12] , [17] Among 1,035 patients with PE and systolic BP >90 mm Hg in the International Cooperative Pulmonary Embolism Registry (ICOPER), the presence of RV hypokinesis was associated with a nearly twofold risk of death (hazard ratio, 1.94).[12] A systematic review of 12 studies of RV dysfunction in normotensive patients with PE suggested that RV dysfunction as assessed by echocardiography and spiral CT scan or elevated cardiac biomarkers is associated with a higher risk of short-term mortality;[8] the OR for short-term mortality based on echocardiography and spiral CT scan studies was 2.4 (95% CI, 1.3-4.4). A meta-analysis of 20 studies in patients with acute PE showed that high levels of troponins were associated with a high risk of short-term death compared with normal levels (OR, 5.24; 95% CI, 3.28-8.38).[10] Separate analysis of the seven studies that only included normotensive patients still showed an association between elevated troponin levels and mortality (OR, 4.98; 95% CI, 2.64-9.39).[10] Similarly, a metaanalysis of 13 studies in patients with acute PE demonstrated that high BNP or N-terminal-proBNP levels were at a higher risk of a complicated in-hospital course (OR, 6.8; 95% CI, 4.4-10) and 30-day mortality (OR, 7.6; 95% CI, 3.4-17).[9] In contrast, a review of 157 patients in the Prospective Investigation of Pulmonary Embolism Diagnosis (PIOPED) II study with stable BP and RV enlargement (measured on CT pulmonary angiogram) showed no difference in the in-hospital rate of death from PE or all-cause mortality between 78 patients with RV enlargement and 79 patients without RV enlargement.[17] Bova and colleagues[6] prospectively evaluated the usefulness of six prognostic markers for predicting inhospital adverse events related to PE and 3-month mortality in 201 consecutive patients with acute PE and normal BP. Only one patient (0.5%) died of PE during hospitalization, and inhospital and 3-month all-cause mortality were 2% and 9%, respectively. None of the prognostic markers (RV dysfunction, troponin I, BNP, a validated clinical score, hypoxemia, or D-dimer) predicted the primary study outcome, which was in-hospital PE-related death or clinical deterioration, suggesting these prognostic markers are not useful for risk stratification of normotensive patients with acute PE and should not be used to select patients for aggressive treatment. The most convincing reason for not recommending thrombolysis for patients with submassive PE is that no study has shown a mortality benefit from doing so, and thrombolysis carries a significant risk of bleeding with a reported fatal hemorrhage rate of 2%. [18] , [19] Although thrombolysis is associated with more rapid clot resolution and more rapid improvements in RV function and mean pulmonary artery pressure, these short-term benefits do not result in any longterm hemodynamic improvement. [20] , [21] , [22] , [23] In fact, after 1 week, the percentage of lung perfusion resolution in patients treated with heparin is similar to that in patients treated with thrombolysis.[20] Likewise, echocardiographic findings were similar after 7 days in patients treated with heparin compared with those treated with a thrombolytic agent.[22] Results of the clinical trials that compared treatment of acute PE with thrombolysis with treatment with anticoagulation failed to show any benefit of thrombolytic therapy. [24] , [25] , [26] A meta-analysis of 11 randomized trials comparing thrombolytic therapy with heparin in 748 patients showed no significant difference in mortality or major bleeding between groups.[24] A subgroup analysis of trials that included hemodynamically unstable patients with acute PE (five trials) did show a significant reduction in PE or death in patients treated with thrombolysis compared with heparin. However, no benefit was seen in the six trials that excluded unstable patients. A meta-analysis of all clinical trials directly comparing recombinant tissue plasminogen activator with heparin in hemodynamically stable patients with acute PE was published in 2009. Five studies involving 464 patients were included. No difference in death related to PE or PE recurrence was seen, even in the subgroup of patients with RV dysfunction.[25] An updated Cochrane Review concluded that thrombolytics compared with heparin did not reduce death or PE recurrence in patients with acute PE.[26] Recently published guidelines have indicated similar conclusions. In the ninth edition of the Antithrombotic Therapy and Prevention of Thrombosis: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines, the expert panel reviewed 13 randomized trials that compared thrombolytic therapy alone to anticoagulant therapy alone and concluded that thrombolysis may be associated with a reduction in mortality and recurrent PE but is associated with an increase in major bleeding.[5] Based on the poor quality of the evidence, the panel issued a weak recommendation for thrombolysis in patients with acute PE associated with hypotension who do not have a high risk of bleeding. They reasoned that the high mortality of patients in shock from acute PE justifies the risk of fatal bleeding even if the efficacy of thrombolysis is modest. In most patients with PE without hypotension, the panel recommends against thrombolytic therapy. They do make allowance for physician judgment, however, through the following recommendation: “In selected patients with acute PE not associated with hypotension and with a low risk of bleeding whose clinical presentation or clinical course after starting anticoagulant therapy suggests a high risk of developing hypotension, we suggest administration of thrombolytic therapy.”[5] The panel recognizes that there is no explicit prediction rule to identify this subgroup of patients, however, and suggests that such patients be identified on the basis of clinical instability and failure to improve on anticoagulant therapy. Although parameters of RV size and function, as well as cardiac biomarkers, can assist the physician in selecting such patients for thrombolysis, they do not have sufficient predictive power to serve as independent selection criteria. In fact, the panel advises against their routine measurement. The American Heart Association guidelines parallel those of the American College of Chest Physicians. Fibrinolysis is reasonable in patients with massive PE (systolic BP <90 mm Hg) and acceptable risk of bleeding complications.[4] For patients with submassive PE, their recommendation is as follows: “Fibrinolysis may be considered for patients with submassive PE judged to have clinical evidence of adverse prognosis (new hemodynamic instability, worsening respiratory insufficiency, severe RV dysfunction, or major myocardial necrosis) and low risk of bleeding complications.”[4] The facts are that most patients with submassive PE treated with anticoagulation alone have a low risk of dying (probably <3%).[4] The risk of fatal hemorrhage caused by the administration of a thrombolytic agent is on the order of 2%. [18] , [19] Thus, thrombolysis should be used only in patients with more severe compromise, the need for cardiopulmonary capacity, and at low risk of intracranial hemorrhage. Current methodologies are overly sensitive in classifying many patients without substantial compromise as having “submassive PE,” and thus this population, broadly defined, does not clearly benefit from thrombolytic therapy. To date, not a single randomized controlled trial has identified the subgroup of patients with submassive PE whose survival is improved by the administration of thrombolytic therapy. Until that subgroup is defined, patients with submassive PE are best served when the decision to administer a thrombolytic agent is made on a case-by-case basis by thoughtful, well-informed physicians who carefully weigh the risks and benefits and consider patient preferences, clot burden, acute physiology, comorbidities, need for maximal cardiopulmonary capacity, and bleeding risk. Our position is not contrary to ever giving thrombolytic therapy for submassive PE, but contrary to ever giving thrombolytic therapy for submassive PE as a matter of routine. REFERENCES: 1 Goldhaber SZ, Visani L, De Rosa M: Acute pulmonary embolism: clinical outcomes in the International Cooperative Pulmonary Embolism Registry (ICOPER). Lancet 353. (9162): 1386-1389.1999; Abstract 2 Kucher N, Rossi E, De Rosa M, Goldhaber SZ: Massive pulmonary embolism. Circulation 113. (4): 577582.2006; Abstract 3 Kasper W, Konstantinides S, Geibel A, et al: Management strategies and determinants of outcome in acute major pulmonary embolism: results of a multicenter registry. J Am Coll Cardiol 30. (5): 1165-1171.1997; Abstract 4 Jaff MR, McMurtry MS, Archer SL, American Heart Association Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation; American Heart Association Council on Peripheral Vascular Disease; American Heart Association Council on Arteriosclerosis, Thrombosis and Vascular Biology , et al: Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: a scientific statement from the American Heart Association. Circulation 123. (16): 1788-1830.2011; Abstract 5 Kearon C, Akl EA, Comerota AJ, et al: Antithrombotic therapy for VTE disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 141. (2 suppl): e419S-e494S.2012; Full Text 6 Bova C, Pesavento R, Marchiori A, TELESIO Study Group , et al: Risk stratification and outcomes in hemodynamically stable patients with acute pulmonary embolism: a prospective, multicentre, cohort study with three months of follow-up. J Thromb Haemost 7. (6): 938-944.2009; Abstract 7 Kreit JW: The impact of right ventricular dysfunction on the prognosis and therapy of normotensive patients with pulmonary embolism. Chest 125. (4): 1539-1545.2004; Full Text 8 Sanchez O, Trinquart L, Colombet I, et al: Prognostic value of right ventricular dysfunction in patients with haemodynamically stable pulmonary embolism: a systematic review. Eur Heart J 29. (12): 1569-1577.2008; Abstract 9 Klok FA, Mos IC, Huisman MV: Brain-type natriuretic peptide levels in the prediction of adverse outcome in patients with pulmonary embolism: a systematic review and meta-analysis. Am J Respir Crit Care Med 178. (4): 425-430.2008; Abstract 10 Becattini C, Vedovati MC, Agnelli G: Prognostic value of troponins in acute pulmonary embolism: a metaanalysis. Circulation 116. (4): 427-433.2007; Abstract 11 Konstantinides S, Geibel A, Heusel G, Heinrich F, Kasper W, Management Strategies and Prognosis of Pulmonary Embolism-3 Trial Investigators : Heparin plus alteplase compared with heparin alone in patients with submassive pulmonary embolism. N Engl J Med 347. (15): 1143-1150.2002; Abstract 12 Kucher N, Rossi E, De Rosa M, Goldhaber SZ: Prognostic role of echocardiography among patients with acute pulmonary embolism and a systolic arterial pressure of 90 mm Hg or higher. Arch Intern Med 165. (15): 17771781.2005; 13 Goldhaber SZ: Pulmonary embolism thrombolysis: broadening the paradigm for its administration. Circulation 96. (3): 716-718.1997; Citation 14 Agnelli G, Becattini C, Kirschstein T: Thrombolysis vs heparin in the treatment of pulmonary embolism: a clinical outcome-based meta-analysis. Arch Intern Med 162. (22): 2537-2541.2002; 15 Grifoni S, Olivotto I, Cecchini P, et al: Short-term clinical outcome of patients with acute pulmonary embolism, normal blood pressure, and echocardiographic right ventricular dysfunction. Circulation 101. (24): 28172822.2000; Abstract 16 Kasper W, Konstantinides S, Geibel A, Tiede N, Krause T, Just H: Prognostic significance of right ventricular afterload stress detected by echocardiography in patients with clinically suspected pulmonary embolism. Heart 77. (4): 346-349.1997; Abstract 17 Stein PD, Beemath A, Matta F, et al: Enlarged right ventricle without shock in acute pulmonary embolism: prognosis. Am J Med 121. (1): 34-42.2008; Full Text 18 Levine MN: Thrombolytic therapy for venous thromboembolism. Complications and contraindications. Clin Chest Med 16. (2): 321-328.1995; Abstract 19 Kanter DS, Mikkola KM, Patel SR, Parker JA, Goldhaber SZ: Thrombolytic therapy for pulmonary embolism. Frequency of intracranial hemorrhage and associated risk factors. Chest 111. (5): 1241-1245.1997; Full Text 20 Urokinase pulmonary embolism trial. Phase 1 results: a cooperative study. JAMA 214. (12): 2163-2172.1970; 21 Goldhaber SZ, Haire WD, Feldstein ML, et al: Alteplase versus heparin in acute pulmonary embolism: randomised trial assessing right-ventricular function and pulmonary perfusion. Lancet 341. (8844): 507-511.1993; Abstract 22 Konstantinides S, Tiede N, Geibel A, Olschewski M, Just H, Kasper W: Comparison of alteplase versus heparin for resolution of major pulmonary embolism. Am J Cardiol 82. (8): 966-970.1998; Abstract 23 Fasullo S, Scalzo S, Maringhini G, et al: Six-month echocardiographic study in patients with submassive pulmonary embolism and right ventricle dysfunction: comparison of thrombolysis with heparin. Am J Med Sci 341. (1): 33-39.2011; Abstract 24 Wan S, Quinlan DJ, Agnelli G, Eikelboom JW: Thrombolysis compared with heparin for the initial treatment of pulmonary embolism: a meta-analysis of the randomized controlled trials. Circulation 110. (6): 744-749.2004; Abstract 25 Tardy B, Venet C, Zeni F, Coudrot M, Guyomarc'h S, Mismetti P: Short term effect of recombinant tissue plasminogen activator in patients with hemodynamically stable acute pulmonary embolism: results of a metaanalysis involving 464 patients. Thromb Res 124. (6): 672-677.2009; Abstract 26 Dong BR, Hao Q, Yue J, Wu T, Liu GJ: Thrombolytic therapy for pulmonary embolism. Cochrane Database Syst Rev . (3): 2009;CD004437 Abstract