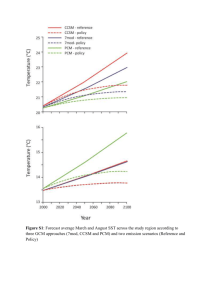
MoU of Action
advertisement

European Cooperation in Science and Technology - COST —————————— Secretariat ------ Brussels, 4 July 2012 COST 4155/12 MEMORANDUM OF UNDERSTANDING Subject : Memorandum of Understanding for the implementation of a European Concerted Research Action designated as COST Action TD1204: Modelling Nanomaterial Toxicity MODENA Delegations will find attached the Memorandum of Understanding for COST Action as approved by the COST Committee of Senior Officials (CSO) at its 185th meeting on 6 June 2012. ___________________ COST 4155/12 1 DG G III EN MEMORANDUM OF UNDERSTANDING For the implementation of a European Concerted Research Action designated as COST Action TD1204 MODELLING NANOMATERIAL TOXICITY MODENA The Parties to this Memorandum of Understanding, declaring their common intention to participate in the concerted Action referred to above and described in the technical Annex to the Memorandum, have reached the following understanding: 1. The Action will be carried out in accordance with the provisions of document COST 4154/11 “Rules and Procedures for Implementing COST Actions”, or in any new document amending or replacing it, the contents of which the Parties are fully aware of. 2. The main objective of the Action is to produce Quantitative Nanostructure-Toxicity Relationships (QNTR) models for nanomaterials, through the coordination of interdisciplinary collaborations of different stakeholder parties. 3. The economic dimension of the activities carried out under the Action has been estimated, on the basis of information available during the planning of the Action, at EUR 36 million in 2012 prices. 4. The Memorandum of Understanding will take effect on being accepted by at least five Parties. 5. The Memorandum of Understanding will remain in force for a period of 4 years, calculated from the date of the first meeting of the Management Committee, unless the duration of the Action is modified according to the provisions of Chapter V of the document referred to in Point 1 above. ___________________ COST 4155/12 2 DG G III EN TECHNICAL ANNEX A. ABSTRACT AND KEYWORDS Nanotechnology produces engineered nanomaterials (ENM) having new or enhanced physicochemical properties in comparison to their micron-sized counterparts. Some of these properties, like the high surface area to volume ratio, make them potentially dangerous to humans as shown by research in NANOTOXICOLOGY. To promote the development of a new generation of ENM that are SAFE-by-DESIGN, an understanding of the relationship between the ENM STRUCTURE and the biological ACTIVITY is needed. In this context, Quantitative Nanostructure-Toxicity Relationships (QNTR) computational modelling technique is an effective alternative to experimental testing since it enables the prediction of (eco)-toxicological effects based on ENM structure only. The construction of QNTR model requires the integration of expertise of nanomaterial scientists, (eco)-toxicologists, and modellers from academia, regulatory agencies and industry. Therefore, a network for trans-disciplinary cooperation is needed. Thus, this COST Action (MODENA – Modelling Nanomaterial Toxicity) will promote and realise through the coordination of these inter-disciplinary collaborations of different parties with the ultimate aim of producing QNTR models for ENM. The important benefits from MODENA include: (i) the development of a new generation of SAFE-by-DESIGN ENM; (ii) the effective reduction of animal testing and (iii) The creation of transparent, validated and rigorous QNTR tools for regulatory purposes in the field of nanotoxicology according to OECD principles. Keywords: Key Words: Nanotechnology, Nanoscience, Nanotoxicology, QSAR, QNTR, Database, Human Health, Toxicology, Ecotoxicology, Nanomedicine B. BACKGROUND B.1 General background Nanotechnology is recognised as one of the most important new technologies of the 21st century. The global investment in nanotechnology from all public sources for 2008 exceeds $7 billion. The market size for nanotechnology is expected to grow to over $3 trillion by 2015 with an estimate of 50,000 products containing engineered nanomaterials (ENM). Nanotechnology promises new materials for industrial applications by having new or enhanced physico-chemical properties that are different in comparison to their bulk or micron-sized counterparts. However, as in all industrial applications, the potential exposure of humans and the environment to these materials is inevitable. COST 4155/12 TECHNICAL ANNEX 3 DG G III EN As these materials go through their life-cycle – from development, to manufacture, to consumer usage, to final disposal – different human groups (workers, bystanders, consumers), animal species (e.g. worm, fish or humans through secondary exposure) and environmental compartment (air, soil, sediment, ground and surface water) will be exposed to them. Given the current pace of development of ENM based applications and the current severely reduced time to market for new ENM based products, risk assessors are challenged with the need to assess possible adverse effects on strongly reduced timescales. A growing body of evidence has shown a range of toxic effects from ENM, suggesting that even their low mass exposure will result in a risk to human health or the environment. Furthermore, the toxicity of ENM can be attributed to some of their physico-chemical properties such as surface area, charge or reactivity. Therefore there is a clear need for a better understanding of the relationship between ENM properties and the adverse responses which they evoke in living organisms. Clearly understanding this relationship will greatly help in designing future ENM with the ‘safe by design’ approach. Identifying and quantifying the relationship between ENM properties and the biological responses can be done by using Quantitative Nanostructure-Toxicity Relationships (QNTR) models, which represent the extension to ENM of the well-known Quantitative Structure-Toxicity Relationships (QSAR) models and can be used by risk assessors and other stakeholders to effectively and quickly assess possible risk without the need of extensive additional testing. With this COST Action, it will be possible harmonise all the scientific advances that are necessary for the future use of reliable QNTR models in regulatory contexts. Indeed, the European Union (EU) has set ambitious plans for the future of Nanotechnology. Accordingly, the different member states and FP7 have made calls for proposals to respond to these goals. This COST Action on the modelling of the toxicity of ENM by means of QNTR will provide a robust mechanism for coordinating the research of leading-edge academic groups within Europe in this area, focusing activities on key targeted areas rather than using the normal fragmented approach. The involvement of industrial partners and of research groups will also promote technology transfer between research organisations and industry and access to essential data needed to advance QNTR. This Action has its strength in non-competitive research, in flexible multinational cooperation and in solving cross-discipline challenges with the help of a multidisciplinary approach. It will add synergy and added value to European research cooperation. COST 4155/12 TECHNICAL ANNEX 4 DG G III EN B.2 Current state of knowledge QTNR is ideal for a rapid assessment of toxicological hazards posed by ENM. The usefulness of quantitative relationships that can model the impact of structural changes on toxicological endpoints has been extensively proved in the pharmaceutical industry over several decades. Moreover, current toxicological regulation, such as the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH), strongly promotes the use of these predictive modelling. Available data are so far insufficient or inadequate to meet this need. This is because research to determine impacts of ENM on diverse biological systems, although essential for assessing their hazard, is timeconsuming and expensive, and has ethical implications when animals are used. In silico methods for predicting biological effects of nanomaterials play an important, complementary role to that of experimental researches. Due to the complexity of interactions of ENM with living organisms and the increasing use of high-throughput content screening methods to generate large in vitro datasets, risk analysis, statistical modelling and machine learning methods (e.g. neural networks) are becoming methods of choice. They have been applied successfully to development of pharmaceuticals and crop protection agents over the past several decades. QSAR methods are increasingly being used by regulatory agencies for chemical risk assessment. They have also been applied more recently to modelling the properties of materials, including nanomaterials. Although QSAR techniques have only started to be used to predict biological effects of nanomaterials they have shown encouraging initial results. However, nanomaterials present significantly different obstacles to modelling compared to drugs and industrial chemicals because their specific properties, such as size, shape, surface area, surface reactivity, so QNTR modelling are therefore needed. Issues pertinent to the development of computational methods for modelling nanomaterial properties and their biological effects will be central to this COST Action, together with developments in research that are required if the regulation of nanomaterials is to be assisted by computational tools within the next decade. Application of QNTR involves several steps. Firstly, chemical or structural properties of ENM are represented by mathematical objects called descriptors, many of which can be calculated or measured. COST 4155/12 TECHNICAL ANNEX 5 DG G III EN Examples of descriptors suitable for ENM include particle size, shape and surface area, ionisation potentials of metals, heats of formation of metal oxide clusters, band gaps, zeta potentials, and physicochemical properties (e.g. lipophilicity, hydrogen bond donor or acceptor strength) of molecules covalently bound to ENM surfaces. Secondly, using additional mathematical techniques, subsets of descriptors are chosen that are most likely to relate to the biological property (e.g. cell apoptosis, metabolism, or signalling pathway modulation) being modelled. Statistical modelling or machine learning methods, for example neural networks, generate mathematical models linking descriptors to biological activity. Finally, the model’s robustness and ability to predict properties of new materials is assessed by statistical cross-validation techniques, or by predicting properties of materials in a test set not used to develop the model. It is therefore clear that the QNTR models require considerable amount of data. Thus, identifying, collecting and harmonising different datasets are important to the construction of QNTR models. QNTR is ideal for rapidly exploring the effects of a large number of variables in complex scenarios and has proven very useful in the pharmaceutical industry over several decades. Many important pharmaceutical products now on the market were discovered and optimized in the past following this QSAR approach (e.g. sulfamethoxazolo, cefalotin and analogs, and captopril). Examples of statistical or machine learning methods used in the context of QNTR are neural networks, decision trees, and support vector machines which are aimed to model the relationships between the molecular structure and the biological properties. These are well validated and tested methods that have been improved substantially over the past decade by incorporation of recent developments in mathematics and statistics. QNTR can be used to form “categories” of similar structures whose physicochemical and toxicological properties follow a regular and predictable pattern that can be easily and confidently adopted as toxicological evidence for hazard assessment. The regulatory use of chemical categories is promoted by international regulatory bodies because it provides a sound basis for effective communication among all the stakeholders who will give different weight to various decision criteria such as cost, efficacy and safety. The methods are robust and intrinsically applicable to modelling a wide range of properties including material properties and biological effects. COST 4155/12 TECHNICAL ANNEX 6 DG G III EN Preliminary work demonstrates that QNTR shows considerable promise for modelling ENM toxic effects but scientific research on specific aspects of QNTR modelling (e.g. pristine ENM versus ENM in biological environments) is still in their infancy and needs to be harmonised among different disciplines. QNTR are also useful for predicting toxic effects of new ENM based on their materials properties and for classifying ENM according to common properties or common biological endpoints. Ultimately, the predictive power of QNTR will lead to considerable reduction in the use of animal experimentation in the safety and hazard assessment of ENM. B.3 Reasons for the Action This Action will contribute to the promotion of European co-operation between scientists from different COST countries. Some of the participants were actively involved in organising and promoting the COST sponsored QNTR workshop in Maastricht (2011) where it emerges that the complexity of the studied phenomena the participation of scientists, from different disciplines, is an indispensable issue for an effective progress of QNTR. QNTR will bring together, with to the crucial support of computer sciences (database, data mining) and mathematics, the considerable knowledge in Metrology, exposure sciences, mammalian toxicology and eco-toxicology and advanced material science. This multi-disciplinary collaboration will put Europe at the forefront of ‘SAFE-by-DESIGN’ Nanotechnology, a topic with far reaching economic and environmental impact. Furthermore, the development of QNTR as a tool for hazard assessment of ENM will also be useful for regulatory agencies and industry while permitting to rationalise knowledge in advance material science and (eco)-toxicology. The contribution of QNTR towards the reduction in animal experimentation for regulatory purposes is especially important. Finally, this co-operation is also an opportunity to involve and train young researchers by world class scientists to create the critical mass for a sustainable future research in QNTR. This will also strengthen capacity building and training programs to assist a harmonised scientific approach. COST 4155/12 TECHNICAL ANNEX 7 DG G III EN B.4 Complementarity with other research programmes Nanotechnology is central to the Economic Agenda of the European Union. The 7th Framework of the EU has devoted a nanomaterials process (NMP) platform to promote research in Nanotechnology which includes the research programmes on the safety of ENM. Of interest are the recurrent Seventh Framework Programme (FP7) calls from the NMP platform on modelling the toxicity of nanomaterials. This strategy is also being pursued in many European countries in their national research and development programmes. QNTR modelling is also of interest to international regulatory bodies and has been in included as a topic for inclusion in the Steering Group 7 (SG7) discussion on alternative tests for ENM. C. OBJECTIVES AND BENEFITS C.1 Aim The main objectives of the COST Action are: (i) to create and implement a road map for the development of reliable QNTR models and associated tools; (ii) to bring together the expertise of several scientific communities that currently lack interaction so that they will enable fast progress in developing generic approaches to the use of QNTR techniques; (iii) to train of the new generation of scientists by providing a pool of unique European and international acclaimed experts. The fulfilment of these objectives will impact positively the future career of young scientists by providing state-of-the-art network infrastructures. Furthermore, the Action believes that regular workshops and schools will also contribute substantially to sharing the expertise of scientists working on the development of QNTR with groups in industry and governmental regulatory agency and will thus enable a rapid and operational knowledge transfer across the nanosafety and nanomedicine stakeholders in order to create critical mass. In this respect it should explicitly be noted that the field of QNTR and its applications is multidisciplinary by definition. COST 4155/12 TECHNICAL ANNEX 8 DG G III EN C.2 Objectives To reach the main objectives, the following secondary goals have been defined: i) To create a European network of experts, from Academia, National/European regulatory agencies, European Technology platforms and clusters and Industry, working to promote the exchange of expertise and information between these communities. The specific groups are for: ii) 1. Synthesis and characterisation of ENM, 2. Mammalian toxicology, 3. In vitro toxicology 4. Ecotoxicology, 5. System biology, 6. Informatics: Establishment of databases and modelling, 7. Risk assessors To train for the use of QNTR existing models designed and developed as part of research programmes already funded at the national and European level (e.g. the FP7 NMP programme). iii) To establish a strategy for further development of QNTR and promoting their use in nanosafety and nanomedicine research and industry. C.3 How networking within the Action will yield the objectives? Objectives will be achieved through exactly planned operations like: 1. Every participating country has their own national support project relevant to the development of QNTR methods that can be used for the Action. COST 4155/12 TECHNICAL ANNEX 9 DG G III EN 2. The main resources (manpower, equipment, infrastructure) and expertise needed to achieve the goals of the Action are already available at the participating researchers (institutes, universities and private sector collaborators) and put at the disposal of the Action, which means effective, immediate start for the generation of results. 3. Collaborators have quality-based working systems in required level certifying the outcomes and concepts. 4. There is systematic plan to achieve aims through multidisciplinary and scientific experts network which have in their use the latest methods and tools. 5. The experts know very well the prevailing scientific stage and its actual problems and what needs to be developed. 6. Among the participants there are researchers and risk assessors with experience in computational models for regulatory purposes therefore ensuring that QNTR approaches will be pertinent for hazard assessment according to the standards required by governmental bodies and agencies operating within (eco)-toxicological regulations. 7. The participants have extensive experience in managing and developing scientific programs at European and International level. C.4 Potential impact of the Action As explained above, this COST Action has the potential to exert a positive impact in many ways 1. Economically by promoting the development of new ‘safe by design’ ENM 2. Regulatory by allowing risk assessors to rapidly take informed decisions on the possible risks of newly developed ENM 3. Scientifically (by overcoming all the scientific barriers and experimental fragmentation leading towards the adaptation of the QSAR paradigm to ENM) 4. Ethically (by refining, reducing and replacing the use of experimental animals). COST 4155/12 TECHNICAL ANNEX 10 DG G III EN Some additional benefits linked to this Action are as follows: 1. Predictions from QNTR can be used for the classification and labelling of ENM in the framework of toxicological regulations such as the European regulation REACH and international regulatory body activities. 2. The participating groups, the larger scientific communities and many national and EU funded programmes will benefit from advances in this field and the synergistic effects generated by sharing of expertise and of teaching young scientists. C.5 Target groups/end users Results of the Action will not only be a significant benefit for scientists researching on the different disciplines involved in the Action (e.g. materials science, (eco)-toxicology) but on European policy makers who in view of the results will be able to launch new initiatives to develop safer ENM and nanotechnology, and the public in general, who are really concerned about the potential health problems and environmental effects of ENM. For this reason special efforts will be dedicated to the assessment and communication of all the inherent uncertainties that could prove to be crucial during decision-making on the basis of QNTR. In this respect, the COST Action MODENA, which coordinates the scientific innovations within Europe in this important area, has the potential for economic, environmental and societal benefits. From this point of view, results of the Action have the potential for industrial and societal benefits; not only in the sustainable, intelligent and inclusive Nanotechnology sector but positive effects will also be observed in many other areas such as ethics and regulatory policy making. COST 4155/12 TECHNICAL ANNEX 11 DG G III EN D. SCIENTIFIC PROGRAMME D.1 Scientific focus This Action will focus on the development and implementation of QNTR models. The three main issues for QNTR are: 1. Defining the biologically relevant entity Unlike chemicals, the surface properties of nanomaterials can change in an environment-specific manner. When they are taken up by humans a nano-bio interface (corona), consisting mainly of proteins in the systemic circulation and of phospholipids in the lung, is generated. In the natural environment, ENM may be coated with ions, proteins, or other molecules like humic substances, depending on whether they are in a stream, soil or other compartment. Protein or phospholipid binding to ENM in biological fluid is not a static process, being characterised by continuous association and dissociation events that reach equilibrium, whereupon continued exchange does not affect the corona composition. The composition of the protein corona may be considered a fingerprint of a specific nanomaterial in a given compartment. However, if the nanoparticle moves from one compartment to another (e.g. from the lung to blood), the corona will be modified and changing the ENM properties over time. Another dynamic process occurring in biological systems is the exchange between the agglomerated and the dispersed forms of ENM, which may change as the environment changes and this is currently poorly understood. In addition, potential dissolution of ENM in certain environments may be modified by the composition and surface coverage of the corona. Modelling and prediction of the biological effects of nanomaterials requires a better understanding of these dynamic processes through experiments. High throughput experimental methods can give this information through rapid measurements of binding affinities of many potential corona components and nanoparticle types, potentially many thousands of experiments. This will allow the generation of QNTR models linking nanoparticle and adsorbent properties to the resultant nanoparticle corona composition that can be used to predict corona formation more broadly. The data can also parameterise and validate competitive binding models that predict corona properties in different environments. COST 4155/12 TECHNICAL ANNEX 12 DG G III EN ENM may enter the human body by various routes, such as the mouth, nose, skin or eyes. Methods using radioactive tracers and magnetic resonance imaging can track movement of ENM around the body. Other techniques such as electron and confocal microscopy can image ENM in cells. New experimental techniques like scanning near-field ultrasonic holography and Focused Ion Beam (FIB) allow much-improved imaging of the interaction of ENM with cells, increasing knowledge of uptake and fate, as well as, effect on cell function. These methods require further development to allow in vivo tracking of ENM at the typical concentrations in the body during likely occupational exposure, to determine their kinetics of transport, and fate. Once the nanoparticle characterisation, corona composition, and translocation data described above become available in sufficient quality and for a wide range of environments and nanoparticle types, we will gain the ability to understand and predict the bioactive species for ENM in many environments. To this end, QSAR approaches can play a valuable role. They can model and predict the in situ forms of ENM, and the time- and environment-dependent changes in the nanoparticle composition from the high throughput experimental data. 2. Selection of the right assays Clearly, generation of large volumes of in vivo data is not possible from ethical or cost perspectives. However, regulators need to estimate the potential hazard of a given nanomaterial in the workplace, home, or environment. Consequently, it is essential to understand the major mechanisms of toxicity for nanomaterials and define relevant in vitro testing procedures (assays) that can measure the toxic effects of the nanomaterials and that correlate well with the effects of nanomaterials in vivo. The selection of biological properties measured will almost certainly be end-use dependent, depending on whether human or environmental effects are the subjects of concern. As with nanoparticle characterisation, and corona composition measurement, high throughput and high content screening (measuring several biological responses in cells simultaneously) in vitro toxicity assays developed for the pharmaceutical industry can be adapted for ENM. This will greatly increase the amount of nanotoxicity data that can be generated for use in modelling and to improve our knowledge of mechanisms of toxicity of ENM. COST 4155/12 TECHNICAL ANNEX 13 DG G III EN 3. Modelling the complex nanomaterial-biology interactions In QNTR methods, the bioactive form of ENM for a given environment is converted into suitable descriptors such as size, ionisation potential of metals, or number and types of functional groups in surface modified ENM. In vivo or relevant in vitro, data together with physicochemical descriptors that can effectively describe crucial structural properties that modulate (eco)-toxicological endpoints are then used to train these models. For instance the mathematical representations of an ENM (i.e. descriptors) can be applied to a neural network input layer and with a suitable number of hidden layers the network generates a predicted value for the biological response at the output layer. This is compared with experimentally measured biological responses for each nanoparticle in the data set and the error used to modify the weights in the neural network to improve the predictions. Once validated (i.e. with international regulatory body principles for QSAR validation), the model can be used to predict properties of new nanomaterials, or to elucidate biological mechanisms and processes. The large volumes of data that the high throughput experimental methods referred to above will allow the development of QNTR models for properties such as corona composition for specific environments, in vitro. Such models will firstly allow in vitro responses of new nanomaterials to be predicted and then allows experimental work to be focused more effectively by identifying additional properties of particular concern. QNTR modelling of large data sets therefore requires cycles of iteration to be established between experimentalists and modellers that will allow predictions to be tested and subsequently, models refined. The refined models will be better predictors of biological responses to new nanomaterials. Ultimately, in vivo effects of ENM are the most important for regulatory purposes, although they are the most expensive and difficult to obtain by experiment. The combination of results from in vitro assays (or predictions from QNTR models of in vitro assays) and nanomaterials descriptors such as size, shape, composition, zeta potential, elemental and molecular properties and corona composition constitute nanoparticle ‘fingerprints’ that can be used to derive QNTR models of in vivo activity. Needless to say, there is a clear need for the construction of database to store and retrieve data for modelling purposes. These databases can be considerably large and complex due to the types of data stored. Data mining techniques will be needed to identify trends and patterns in these complex datasets. COST 4155/12 TECHNICAL ANNEX 14 DG G III EN Two of the major challenges to applying QNTR methods to modelling biological properties of ENM are: 1. insufficient experimental data on corona composition and in vitro and in vivo effects (discussed above) 2. the need for better ‘nanoparticle-specific’ descriptors. Nanomaterials differ substantially in structure from small organic molecules for which the existing descriptors were developed. Although existing descriptors work well for modelling some nanomaterials, it is clear that further research is required to generate nanomaterials-specific mathematical descriptors. D.2 Scientific work plan methods and means The Action’s scientific program is divided into three Working Groups (WG) focusing on major disciplines related to the development and use of QNTR. The Management Committee (MC), in coordination with WG leaders and the Short-Term Scientific Mission (STSM) Coordinator will create a Steering Committee for an efficient administrative and scientific management. Indeed, the development of QNTR models requires the synergy among three areas of expertise: physical Chemistry (WG1), (eco)-Toxicology (WG2) and Modelling (WG3). With to the harmonisation of all the ongoing research in these fields, MODENA will provide an optimal network and “knowhow” for a successful interaction among them. The implementation and monitoring of the strategic vision exposed in this COST Action will be overseen by the Steering Committee which will be created in coordination with WG leaders and the STSM manager. COST 4155/12 TECHNICAL ANNEX 15 DG G III EN WG1 Synthesis and Characterisation of ENM This WG is responsible for the MODENA activities on synthesis and metrology of ENM. The specific objectives of WG1 are to study the synthesis of ENM with controlled composition, size, area and nano-texture and to develop of strategies to immobilise ENM in matrices, on substrates with minimum effect on the desired properties and surface reactivity and identify the relevant datasets for QNTR modelling. This rigorous approach will permit the development of QNTR models on the basis of carefully characterised and selected ENM. The knowledge derived from QNTR would help in designing new ENM that would minimise their potential hazard (i.e. the SAFE-by-DESIGN concept). This information is the basis to assess structural, physicochemical and toxicological features that will be related to property and toxicity. WG2 Toxicity of ENM This WG is responsible for the MODENA activities on toxicity and eco-toxicity of ENM by identifying the relevant datasets. The specific objectives of WG2 are to study and assess the toxicity and eco-toxicity prepared of the ENM prepared inWG1. WG1 and WG2 will interface to assess relationships between toxicity parameters and structural, chemical and reactive features which will deliver preliminary models for WG3. The choice of the (eco)-toxicological studies will be taking regulatory requirements into account at an early stage in order to deliver models of practical interest for all the stakeholders. Moreover, a direct interaction among (eco)-toxicologists and modellers will enable the characterisation of toxicological dataset that are optimised for QNTR modelling. Indeed, thus far, (eco)-toxicological studies have been conducted without taking into consideration criteria that can permit the elaboration of effective QNTR modelling. In the context of this WG, (eco)toxicologists will have the unique opportunity to strengthen the interface between human and environmental hazard assessment of ENM. COST 4155/12 TECHNICAL ANNEX 16 DG G III EN WG3 QNTR modelling and Database This WG is responsible for MODENA activities on identifying and quantifying the relationship between ENM properties and the biological responses using the pertinent physiochemical descriptors. It will also collect and organise the datasets identified in WG1 and WG2 by means of databases. Special attention will be paid to the description of chemical interactions between surfaces and biological molecules, in order to develop an insight into the rationale behind QNTR. This analysis will enable the definition of specific physicochemical descriptors that are able to capture specific properties of ENM as opposed to bulk materials. MODENA will also establish a close collaboration between modellers and (eco)-toxicologists so that mechanistic considerations on toxicological mode of actions will be taken into account when interpreting the logic underlying the developed QNTR models. This approach will enhance the epistemological interpretation of the final outputs of MODENA while enabling the definition of mechanistic chemical categories that are currently used in the framework of toxicological regulations to fill data gaps. This WG will also work closely with end users and risk assessors in industry and regulatory agencies. In all WG, the following tasks are recurrent: 1. Review state-of-the-art development in its target topic (WG1, WG2 and WG3); 2. Contribute to Workshops and Training Schools and identify relevant European and international scientists for invitation to these events; 3. Identify and obtain relevant datasets, assess and identify the relevant uncertainties that could impact on the predicted endpoints; 4. Contribute to the MODENA website; 5. Contribute to the scientific management of MODENA through progress reports; 6. Contribute to the strategy for the development of QNTR. 7. Contribute to the identification, recognition and dissemination of the established QNTR models as animal replacement (based on the 3R’s – Reduction, Refinement and Replacement) COST 4155/12 TECHNICAL ANNEX 17 DG G III EN E. ORGANISATION E.1 Coordination and organisation The Action organisations will follow the general COST rules described in the "Rules and Procedures for Implementing COST Actions". The following positions will be created and named at the first MC meeting during the Kick-off Workshop. These positions will be preferably assigned to ESR (Early Stage Researchers) who will get directly exposed to science management and coordination, giving them more visibility. Gender and age balance will be sought in every organisation aspect of the Action, while keeping emphasis on excellence. 1. The MANAGEMENT COMMITTEE (MC) Chair and Vice-Chair will coordinate the Action. 2. The Action will have three scientific WORKING GROUPS (WGs). Each will have its WG leader. 3. A SHORT-TERM SCIENTIFIC MISSION (STSM) COORDINATOR will be established. 4. A DISSEMINATION COORDINATOR will be established, who will take care of publicising the scientific results of the Action, through its website and in coordination with other initiatives, like elaboration of special issues in a journal or a dedicated booklet. An official website will be developed to foster communication (intranet site) and dissemination (regular web), as commented in section H. 5. For a dynamic management, a STEERING COMMITTEE comprising members of the MC, Chair, Vice-Chair, WG leaders and STSM Coordinator and Dissemination Coordinator will be established. It will coordinate events such as conferences/workshops, dissemination activities, Training Schools, Special issues. Regular meetings of the Steering Committee will be held on telephone-conference or Internet basis for an efficient inexpensive management of the Action. These decisions and their reasoning will be sent to the MC for approval, via “written procedure” using Internet facilities. In any case, major issues will be handled directly by the Management Committee at its annual meeting. A KICK-OFF meeting will be held at the beginning of the Action in order to crystallise Action details, publicise the Action and call for new members to join the defined WGs. A first MC meeting will then be held to elect the MC Chair, Vice-Chair, WG leaders, STSM and Dissemination Coordinators. COST 4155/12 TECHNICAL ANNEX 18 DG G III EN E.2 Working Groups The Action will coordinate a multidisciplinary collaboration and networking among complementary groups. Continuous coordination and interaction of the research among WGs will be fostered by Training Schools and STSM involving all WGs. WGs will have annual meeting, celebrated during the Annual Workshop/MC meeting, to optimise travel budget, so that most budget effort may concentrate on STSM’s and Training Schools, as the key instruments to implement coordination of research activities among laboratories. Additional WG meetings will happen by taking advantage of other events, like a conference at which WG members may convene, and essentially via telephone conference and Internet means. This Action stands on three WGs, as described in section D.2, focusing on major disciplines related to different aspects of QNTR: WG1 on Synthesis and Characterisation of ENM; WG2 on Toxicity of ENM and WG3 on QNTR modelling and Database. E.3 Liaison and interaction with other research programmes The Action will establish collaboration with Industry organisations. The Action will also be of interest to other COST Actions, such as: 1. TD1002 | European network on applications of Atomic Force Microscopy to NanoMedicine and Life Sciences (AFM4NanoMed&Bio). 2. MP0903 | Nanoalloys as advanced materials: from structure to properties and applications (NANOALLOY) 3. TD1007 | Bimodal PET-MRI molecular imaging technologies and applications for in vivo monitoring of disease and biological processes 4. FA0904 | Eco-sustainable food packaging based on polymer nanomaterials COST 4155/12 TECHNICAL ANNEX 19 DG G III EN 5. CM1102 | Multivalent Glycosystems for Nanoscience - MultiGlycoNano 6. TD1105 | European Network on New Sensing Technologies for Air-Pollution Control and Environmental Sustainability – EuNetAir 7. MP0701 | Composites with Novel Functional and Structural Properties by Nanoscale Materials (Nano Composite Materials-NCM) 8. CM1104 | Reducible oxide chemistry, structure and functions 9. FA0904 | Eco-sustainable food packaging based on polymer nanomaterials Communications with these Actions will be established as soon as this Action becomes operative. Suitable relations include the invitation of members of these Actions for ‘Inter-Action’ lectures or the formation of joint workshops. This Action will also be pro-actively in contact with the NMP F7 projects on Nanosafety, specially the projects on the ‘Modelling of the Toxicity of Nanomaterials’. Finally, this Action will be in direct communication with the WG on Database and Modelling of the NANOSAFETY cluster. This Action will also seek to contribute to the European Technology Platforms and Clusters: ETP-Nanomedicine, EPOSS, Nanofuture, Pharmaceutical etc. where QNTR methods will be needed. E.4 Gender balance and involvement of early-stage researchers This COST Action will respect an appropriate gender balance in all its activities and the Management Committee will place this as a standard item on all its MC agendas. The Action will also be committed to considerably involve early-stage researchers. This item will also be placed as a standard item on all MC agendas.The positions described for Coordination and Organisation (E.1) will be preferably assigned to ESR who will get directly exposed to science management and coordination, giving them more visibility. Gender and age balance will be sought in every organisation aspect of the Action, with emphasis on excellence. An important mission for this Action is to rise a new generation of scientists whose training is at the interfaces between medicine, biology, biochemistry, chemistry and physics so that they can efficient understand, describe and handle the relationships between ENM features and their toxicology. For that reason, this Action aims at exposing ESR to this multidisciplinary topic. COST 4155/12 TECHNICAL ANNEX 20 DG G III EN A key element is a multidisciplinary training, not available elsewhere. For such a reason, the Action will organise two Training Schools so that experienced well-known researchers from biology, medicine, chemistry will train ESR with different backgrounds on the concept, methods and technologies available. This will expose ESR to peers, senior researchers and methodologies that will result in synergetic interbreeding of complementary knowledge addressing the same problem: nanotoxicity. This Action will educate a new generation of researchers exposed. During the Training School, the ESR will have chance to be exposed not only to the concept but also to hands-on cases. Debate will be a key element in the Training Schools; ESR will present their needs and their wealth of knowledge. This TRAINING SCHOOL will be funded by the Action to COST members, but it will be open to non-COST members. This will increase the visibility of the Action and help cross-fertilisation. To increase the visibility of these Training Schools, they will be organised by COST in coordination with other agencies, groups or universities particularly relevant in aspects of nanotoxicology. In general, the organisation and coordination of activities like Training Schools, Workshops, WG meeting, and positions defined for the Action management will essentially be assigned to ESR members looking for a gender balance. F. TIMETABLE The Action will run on a 4-year basis. Some key aspects scheduled to occur in these years are summarised below. YEAR #1: The Management Committee (MC) of the Action will be formed at the beginning of the year at a Kick-off meeting. This will increase the visibility of the action since it will be in the frame of a workshop to trigger the visibility of the Action and call for new members to join the different WGs already defined. This will consolidate the WGs. Research details will be better defined during this period. After the first 6 months a first progress assessment will internally be made to nail down research direction details. A Training School will be organised during the first year to expose WG members to complementary disciplines and facilitate communication and multidisciplinary collaboration. COST 4155/12 TECHNICAL ANNEX 21 DG G III EN YEAR #2: Mid-term report (prepared during the annual MC/workshop meeting) will occur this year and the progress of different research lines will be assessed. This time will be used to decide if some activities are to be terminated and others to be promoted. This will be a key year for research progress in different WGs and to foster collaboration among WGs based on the multidisciplinary training delivered by the first Training School. YEAR #3: The MC will meet once; the Working Groups meeting and workshop shall coincide with the MC meeting. Based on the first year Training School, a second one will be scheduled for the 3rd year, attending key formation demands. As in the previous, hands-on activities will be a key part for solid training of ESR members. Sessions will be used to discuss how to implement developments into research, protocols and regulations. The Annual workshop of this year will have particular visibility, probably celebrating it next to a major international event relevant to some aspect of toxicity. Action members and non-members will contribute to this conference, and we will organise a special issue with selected papers to disseminate the state of the art and to show the contribution of our Action to it. YEAR #4: The MC will hold a concluding meeting at the end of the year, during which the Final Report will be finalised. During this year, a Strategic Initiative Workshop shall be organised with the goal to bring leading industry and academia fellows along with policy makers (in Europe, US, Asia, and Oceania) together, and to create a forum to foster implementation of progress and connection in the growing knowledge on Nanotoxicology, ENM synthesis/characterisation and protocols. The workshop will have a final deliverable, in the form of a booklet with key recommendations on Nanotoxicology, progress made in general analysed from the perspective of the Action. It will bring an outlook on quantitative knowledge of nanotoxicology, challenges and recommendations. COST 4155/12 TECHNICAL ANNEX 22 DG G III EN Table 1 summarises the MODENA activities and Timeline. YEAR MONTH ACTIVITY 1st MODENA kick-off workshop and first MC meeting. Appointment of Chair, 1 vice-chair, STSM coordinator, Dissemination coordinator, WG leaders and Steering Committee. Call for WG membership applications 3 Deadline for WG membership applications 4 Definition of WGs, and start-up of activities 9 First TRAINING SCHOOL 12 Activity Report first year 2nd 18 Second MC meeting and MODENA workshop with WG meetings. Assessment on Action progress and research lines and actions to be taken. 24 Mid-term report 31 Second TRAINING SCHOOL 33 Third MC meeting and MODENA workshop with WG meetings 42 STRATEGIC INITIATIVE WORKSHOP 46 Booklet based on conclusion from the Strategic Initiative Workshop 48 Final MC meeting and MODENA workshop with WG meetings 3rd 4th G. ECONOMIC DIMENSION The following COST countries have actively participated in the preparation of the Action or otherwise indicated their interest: DE, ES, FI, FR, IE, IT, NL, SE, UK. On the basis of national estimates, the economic dimension of the activities to be carried out under the Action has been estimated at 36 Million € for the total duration of the Action. This estimate is valid under the assumption that all the countries mentioned above but no other countries will participate in the Action. Any departure from this will change the total cost accordingly. COST 4155/12 TECHNICAL ANNEX 23 DG G III EN H. DISSEMINATION PLAN H.1 Who? The results will be disseminated to three target groups the scientific community (academy and industry), to the policy makers and to the general public. 1. Researchers in medicine, chemistry, biochemistry, biology, and medicine from Academia or Industry. In particular, researchers close to nanotoxicity, nanomaterial synthesis, nanomedicine, modelling and characterisation. 2. Policy makers and agencies in charge of toxicity and public health in Europe, Asia, Oceania, America and International. 3. Public in general. H.2 What? The Action web-site will aim at all target audiences, it will be updated by the Action ́s Dissemination Coordinator. Its contents will cover: - Fundamental on nanotoxicology, fundamentals on nanomaterials, fundamentals on characterisation of nanomaterials and their industrial applications - State of the art and recent developments. - Case studies of general interest. - Link to scientific papers published by the Action - Link to any dissemination activity It will serve as core material for final booklet based on the Action. Target 1. Scientific and Industrial community will be reached via standard means used in the scientific communication: - Articles in journals, reviews and books. COST 4155/12 TECHNICAL ANNEX 24 DG G III EN - Presentations at scientific conferences, international conferences and the Action’s annual Workshop, which will be publicised and open to non-COST members. - Reports on the Action website. - Training Schools, open to non-COST members. Target 2. Policy makers and agencies, will have access to the Action website and will receive information from the Action, flyers will be prepared for their information. These will include place the progress in its social and economic frame. A final Exploratory Workshop will be organised at the end of the Action to trigger visibility of the progress and promote implementation of knowledge developed in the Action. Target 3. General Public. The Website is a key element. In addition, the Action will make: - Articles in science magazines and newspapers. - V and web-based science shows/sites - Radio or TV interviews. - Take advantage of science dissemination activities organised by local governments and science museums. H.3 How? 1. Each Action member will acknowledge COST funding in peer-reviewed papers, conference, website, and general dissemination initiatives. Each Action member will also highlight COST funding at conference presentations and,if possible, say some words about it. 2. COST logo will be at conference presentations and website of each WG laboratory member, with active links to COST general site and this Action website. 3. Action´s Annual Workshop and Training Schools will have maximum visibility in academia and industry, through website, event announcement and alerts at dedicated websites about forthcoming events. Workshops and Training Schools allow direct feedback and interaction. COST 4155/12 TECHNICAL ANNEX 25 DG G III EN 4. Each Action Member at an interview or program in the media will mention COST support. 5. All Action members will contact the members’ university/research centre dissemination agent to use their means and will pro-actively contact press, radio, TV, web media when groundbreaking and general interest developments are achieved. 6. All these initiatives will be reflected on the Action website. ___________________ COST 4155/12 TECHNICAL ANNEX 26 DG G III EN