Colorants and glazes in ceramic industry is using vitreous
advertisement

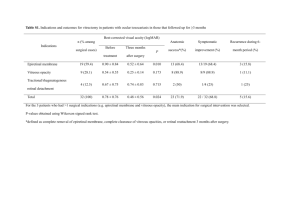
STUDIA UNIVERSITATIS BABES-BOLYAI, PHYSICA, SPECIAL ISSUE, 2003 MICROSTRUCTURAL INVESTIGATIONS OF NEW VITREOUS MATERIAL FOR CERAMIC INDUSTRY Cezara Voica1, Liana Gagea3, E. Indrea2, Simina Dreve2 0and I. Bratu2 1 S.C.CEROC S.A., 1 Treboniu Laurean st., 3400 Cluj-Napoca, Romania 2 National R&D Institute of Isotopic and Molecular Technologies, P.O. Box 700, R-3400 Cluj-Napoca 5, Romania 3 'Babes-Bolyai' University, 1 Kogalniceanu st., Faculty of Chemistry, Cluj-Napoca, Romania Abstract Colorants and glazes in ceramic industry is using vitreous materials named “frits”, containing usually rather large amounts of lead. The necessity of echological and safe products imposed lately the synthesis of new frits lead-free, having as much as possible, the same structure and properties. We report here a new frit-type vitreous lead-free material for fabrication of colorants and glazes in ceramic industry. The chemical composition of the oxidic mixtures for the classical frit F1 and for the new frit and the technological procedures for fabrication are presented. The physical properties as melting point and dilatation coefficient, the specific surface and the optical properties were determined in correlation with the microstructural characteristics. Introduction In traditional ceramics the presence of lead in frits have a very important role: to give the hardness and shining properties and to realise the dispersion of the decoratingcolours in thin layers. Despite this important properties the problems of the us of lead in frits composition (low chemical resistance, pollution, toxicity) imposed the elaboration of new frits, replacing the lead oxyde with other compounds, but keeping the same physical properties. The present work present the preparation of a new frit-type vitreous material with bismuth oxyde, having the capacity to replace the frit with lead and having more convenient physico-chemical properties for use in industrial ceramic. Experimental In order to prepare the new frit-type material usual raw materials from the production process were taken, adjusting the quantities upon the chemical composition and following the usual production process . The important modification of the recipe was the replacement of the lead oxide, PbO, with bismuth oxyde, Bi2O3 , the molar ratios of the chemical compounds in the frit material being presented in table 1. CEZARA VOICA, LIANA GAGEA, E. INDREA, SIMINA DREVE AND I. BRATU Table 1. Molar ratios of the chemical compounds in the frit. Oxydes K2O Na2O CaO MgO ZnO PbO B2O3 Al2O3 Fe2O3 Bi2O3 SiO2 TiO2 Molar ratio leaded frit (1) bismuth frit (2) 0.053 0,433 0,034 0,014 0,165 0.301 1,422 0,201 0,002 3,051 0,002 0,076 0,619 0,049 0,019 0,237 2,033 0,288 0,003 0,206 4,363 0,003 Thermal derivatography To establish the technological firing conditions the thermal analysis of the new material was performed, with a thermal analyser type MOM Q 1500. The two frits have an almost similar thermal behaviour, and results. Between 1600 and 1750 C the same endothermal effects are observed, due to the loss of water from the mixed raw materials. Over 6000 C the effects are very weak, and the loss of weight is low. A calcinations over 8000 C assur ethe melting and the stability of the fondant. The new prepared frit have the loss of wight at calcinations decreased with 1.5% than the leaded frit, at the correspondent calcinations temperatures. The termal analysis curves are represented in fig. 1. Fig. 1. The curves of thermal analysis for the leaded frit (1) and for the new frit with bismuth (2). MICROSTRUCTURAL INVESTIGATIONS OF NEW VITREOUS MATERIAL Thermal microscopy The thermal microscopy analysis was performed on the two frits, using a microscope of high temperatures LEITZ, between 9000 C and 13000 C. The results are presented in table 2. Table 2. Thermal microscopy results for the leaded (1) and bismuth (2) frits. Nr. Temperature 900 1100 1300 (0C) 1 Sample 1 2 1 2 1 2 2 Low-melting690 690 675 690 685 710 point 3 Low-softening 780 790 750 815 780 835 point 4 Melting 870 870 850 880 855 920 5 Softening 90 100 75 125 95 125 interval 6 Melting interval 90 80 100 65 75 85 As it can be seen after 11000 C the thermal behaviour of the two kind of vitreous material is different, and the frit with bismuth is more suited for practical applications because is has larger melting intervals so it assure a better aderence to the ceramic support at a better shining . Structural characterisation The long-range order characteristic for the crystalline state is not occurring in the vitreous state, where the short-range order is typical [1]. For the crystalline structures there are correlations between two atoms disposed at arbitrary large distances one to other. In the non-crystalline solids like glasses there is a local order, a short-range order, characterised only for the first coordination spheres of the component atoms, i.e. at the distances at which the interatomic forces are acting. The local order in glass and crystalline compounds of the same composition has both similarities and differences. The differences are assigned to the loss of long range order, to the deviation from a perfect crystalline structure. The order extension degree can be investigated using the analysis technique of atom electron distribution functions obtained form X-ray scattering measurements. There are also glass systems wherin the local structure is extended at more than some coordination spheres imposing a middle or intermediate range order [2]. The noncrystalline state of a compound can occur in a large structural variety depending on the preparation conditions. This is due to the fact that during the vitreous sample preparation the “freezing” process of the atoms arrangement has an important effect on the atom bonds and atom relaxation energy barriers. Along with the CEZARA VOICA, LIANA GAGEA, E. INDREA, SIMINA DREVE AND I. BRATU neutron scattering the X-Ray scattering from disordered materials is largely used to investigate their local structure [3-7]. The X-ray scattering patterns were obtained by means of standard DRON-3M powder diffractometer, working at 40 kV and 30 mA, and equipped with scintillation counter with single channel pulse height discriminator associated counting circuitry. The Cu K radiation, Ni filtered, was collimated with Soller slits. X- ray scattering patterns were recorded in a step-scanning mode with 2 = 0.100 steps. The analysed samples, disposed as a powered disk with diameter of 12 mm and thickness about 2 mm, were investigated by means of a X ray scattering experiment, using a goniometer in Bragg-Brentano reflection alignment. The structure of the lead oxide, PbO, and bismuth oxyde, Bi2O3 vitrous systems was investigated by analysing the atomic radial distribution function obtained from X ray scattering data using a PEDX program [8]. The computation steps consist first in the determination of radial distribution function 4πr 2∙ρ(r). The preliminary processing of scattering data implies the determination of the total experimental coherent scattering function IT(sk) : IT(sk) = [Iexp(sk) – IF(sk)]·A(sk)/P(sk) correlation function g(r) 25 Si - O 1.69 20 B O-O Si - Si 15 2.93 A 3.21 10 5 0 -5 1,5 2,0 2,5 3,0 3,5 4,0 4,5 5,0 interatomic distance r [A] Fig.2. The atomic pair correlation function g(r) for samples of the lead oxide, A , and bismuth oxyde, B , vitrous systems. The atom pairs correlation function g(r) shows maxima and their position, width and area are determined by the distribution of atom pairs in the structural disordered sample (Fig. 2). The real space distance corresponding to the maxima determined from the data obtained in this study may be compared with results reported for other similar systems [9,10] and allow to identify the atom pairs MICROSTRUCTURAL INVESTIGATIONS OF NEW VITREOUS MATERIAL orderly disposed in the the SiO2 matrix vitrous systems. They are summarised in Table 3. Table 3 Coordination Atom pairs Distance (Å) sphere I Si – O 1.69 II O–O 2.93 III Si - Si 3.21 Inspecting the data obtained for the samples with lead oxide, PbO, and bismuth oxyde, Bi2O3 ,from the analysis of atom pairs correlation function g(r) one remarks the occurrence of Si – O pair as a first coordination formation, well composed at a distance of 1.69 Å. In SiO2 vitrous systems , the basic structural building block is the same as the crystalline silicates, the SiO4 tetrahedron [10]. The distances r between O - O and Si – Si pairs are ranging from 2.6 to 3.5 Å . The bismuth addition to the SiO2 matrix frtits vitreous system preserves the vitreous structure, that evidences the high ability of the precursor glass to accept relatively high Bi 2O3 content without structural changes. References 1. 2. J . Zar z yc k i , Glasses and Vitreous State, Cambridge University Press, 1991 R. E. Yo u n g ma n , S. T . Ha ub ri c h, J . W . Z wa nz i ger , M. T . J a nic k e a nd B . F. C h me l k a, Science 269, 1416 (1995) 3. D. L. P r ic e a nd M. - L. S ab o u n g i , Anomalous X-Ray Scattering from Disordered Materials, Local Structure from Diffraction, eds., S. J. L. Billinge and M. F. Thorpe, Plenum Press, New York, 1998. 4. D. L. P r i ce, M . - L. Sab o u n g i, A. C. B a r ne s , Phys. Rev. Lett. 81,15, 3207 (1998) 5. K. S u z u ya, D. L. P r i ce, C. - K. Lo o n g, S. W . Ma rti n, J. Non-Cryst. Solids, 232-234, 650 (1998) 6. M. No f z, B . H i m me l, T h . Gerb er, F. E hre n tre ic h , J. Non-Cryst. Solids, 143, 191 (1992) 7. B . Hi m me l, J . W ei g el t, T h. Gerb er, M . No fz , J. of Non-Cryst. Solids, 136, 27 (1991) 8. PEDX: a program for radial-distribution function analysis of energy-dispersive X-ray diffraction data from disordered materials (authors: V. Petkov, Department of Solid State Physics, Sofia University, Sofia – 1126, Bulgaria and Y. Waseda, Institute for Advanced Materials, Tohoku University, Sendai 982, Japan). 9. J . S we n so n , L B ö r j e so n , R . L. Mc Gree v y, W . S. Ho we ll s , Phys. Rev. B55, 17, 11236 (1997) 10. R. L.. Mo zzi a nd B . E. W arre n , J. Appl. Crystallogr., 2, 164-172 (1969).