Jianren Mao, M.D., Ph.D - Massachusetts General Hospital
advertisement

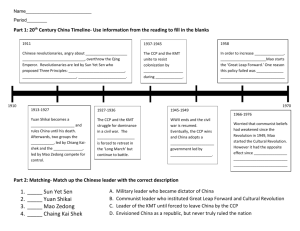
BIOGRAPHICAL SKETCH Provide the following information for the key personnel and other significant contributors in the order listed on Form Page 2. Follow this format for each person. DO NOT EXCEED FOUR PAGES. NAME POSITION TITLE Mao, Jianren Associate Professor eRA COMMONS USER NAME JM7700 EDUCATION/TRAINING (Begin with baccalaureate or other initial professional education, such as nursing, and include postdoctoral training.) INSTITUTION AND LOCATION Suzhou Medical College, China Suzhou Medical College, China Medical College of Virginia, VA DEGREE (if applicable) MD MS PHD YEAR(s) 1983 1986 1992 FIELD OF STUDY Medicine Neuroscience Neuroscience Please refer to the application instructions in order to complete sections A, B, and C of the Biographical Sketch. A. Positions and Honors. List in chronological order previous positions, concluding with your present position. List any honors. Include present membership on any Federal Government public advisory committee. 1987-89 1992-99 1995-99 1999-00 2000-03 20032005 2008 - Assistant Professor, Dept. of Physiology, Suzhou Medical College Assistant Professor, Dept. of Anesthesiology, Medical College of Virginia Anesthesia Resident, Dept. of Anesthesiology, Medical College of Virginia Hospital Clinical Pain Fellow, Massachusetts General Hospital (MGH), Harvard Medical School (HMS) Assistant Professor, HMS; Attending Physician, Anesthesia/Pain Medicine, MGH Associate Professor, HMS; Attending Physician, Anesthesia/Pain Medicine, MGH Director, MGH Center for Translational Pain Research, MGH, HMS Director, MGH Translational Center Investigating Prescription Drug Abuse, MGH, HMS Board-certified in Anesthesia (2000) and Pain Medicine (2001) by the American Board of Anesthesiology; Early Career Scholar Award, American Pain Society, 1997; Reviewer for Sensorimotor Integration Study Section (IFCN), SBIR/STTR Study Section, BRLE, NIDA/SEP.; DOD Biomedical Study Section; Reviewer for Pain; J Neurosci; J Pain; Eur J Neurosci; J Neurophysiol; J Neurochem; Anesthesiology; JPET; Neuroscience; Anesth & Analg; Brain Res; Neurosci Lett; Analgesia; Eur J Pain; Exp Brain Res; Clin J Pain; Life Sci, Cell Res, etc.; Section Editor: Pain Medicine; Associate Editor: Pain; Editorial Board Member: J Pain, J Mol Pain, J Neuropat Pain Palli Care. B. Selected peer-reviewed publications (in chronological order). Do not include publications submitted or in preparation . Mao, J., Hayes, R.L., Price, D.D., Coghill, R.C., Lu, J. and Mayer, D.J.: Post-injury treatment with GM1 ganglioside reduces nociceptive behaviors and spinal cord metabolism in rats with experimental peripheral mononeuropathy. Brain Res., 584:18-27, 1992. Mao, J., Price, D.D., Mayer, D.J., Lu, J. and Hayes, R.L.: Intrathecal MK 801 and local nerve anesthesia synergistically reduce nociceptive behaviors in rats with experimental peripheral mononeuropathy. Brain Res., 576:254-262, 1992. Mao, J., Price, D.D., Mayer, D.J. and Hayes, R.L.: Pain-related increases in spinal cord membrane-bound protein kinase C following peripheral nerve injury. Brain Res., 588:144-149, 1992. Mao, J., Mayer, D.J., Hayes, R.L., Lu, J., and Price, D.D.: Differential roles of NMDA and non-NMDA receptor activation in the induction and maintenance of thermal hyperalgesia in rats with painful peripheral mononeuropathy. Brain res., 598:271-278, 1992. Mao, J., Price, D.D., Coghill, R.C., Mayer, D.J., and Hayes, R.L.: Spatial patterns of spinal cord 14C-2-deoxyglucose metabolic activity in neuropathic rats. Pain, 50:89-100, 1992. Mao, J., Price, D.D., Hayes, R.L., Lu, J., and Mayer, D.J.: Intrathecal GM1 ganglioside and local nerve anesthesia reduce nociceptive behaviors in rats with experimental peripheral mononeuropathy. Brain Res., 584:28-35, 1992. Mao, J., Regelson, W. and Kalimi, M.: Molecular Mechanisms of RU 486 action: A review. Mol. Cell. Biology, 109:1-8, 1992. Mao, J., Coghill, R.C., Kellstein, D.E., Frank, H. and Mayer, D.J.: Calcitonin gene-related peptide enhances substance P induced behaviors via metabolic inhibition: in vivo evidence for a new mechanism of neuromodulation. Brain Res., 574:157-163, 1992. Mao, J., Price, D.D., Hayes, R.L. and Mayer, D.J.: Spatial patterns of increased spinal cord membrane-bound protein kinase C and their relation to increases in neural activity in rats with painful peripheral mononeuropathy. J. Neurophysiol., 70:470-481, 1993. Mao, J., Mayer, D.J. and Price, D.D.: Patterns of increased brain activity indicative of pain in a rat model of peripheral mononeuropathy. J. Neurosci., 13:2689-2702, 1993. Mao, J., Price, D.D., Hayes, R.L., Lu, J., Mayer, D.J. and Frenk, H.: Intrathecal treatment with dextrorphan and ketamine potently reduces pain-related behaviors in a rat model of painful peripheral mononeuropathy. Brain Res., 605:163-168, 1993. Mao, J., Price, D.D. and Mayer, D.J.: Thermal hyperalgesia in association with the development of morphine tolerance: roles of excitatory amino acid receptors and protein kinase C. J. Neurosci., 14:2301-2312, 1994. Mao, J., Price, D.D. and Mayer, D.J.: Mechanisms of hyperalgesia and morphine tolerance: A current view of their possible interactions. Pain, 62: 259-274, 1995. Price DD, Mao J, Frenk H, Mayer DJ. The N-methyl-D-aspartate receptor antagonist dextromethorphan selectively reduces the temporal summation of the second pain in man. Pain, 59:165-174, 1994. Mao, J., Price, D.D. and Mayer, D.J.: Experimental mononeuropathy reduces the antinociceptive effects of morphine: implications for common intracellular mechanisms involved in morphine tolerance and neuropathic pain. Pain, 61:353-364, 1995. Mao, J., Price, D.D., Phillips, L.L., Lu, J. and Mayer, D.J.: Increases in protein kinase C immunoreactivity in the spinal cord of rats associated with tolerance to the analgesic effects of morphine. Brain Res., 677:257-267, 1995. Mao, J., Price, D.D., Caruso, F. and Mayer, D.J.: Oral administration of dextromethorphan prevents the development of morphine tolerance and dependence in rats. Pain, 67:361-368, 1996. Manning, B, Mao, J., Frenk, H., Price, D.D. and Mayer, D.J.: Continuous co-administration of dextromethorphan or MK-801 with morphine: attenuation of morphine dependence and naloxone-reversible attenuation of morphine tolerance. Pain, 67:79-88, 1996. Mao, J., Price, D.D., Zhu, J., Lu, J. and Mayer, D.J.: The inhibition of nitric oxide-activated poly(ADP-ribose) synthetase attenuates transsynaptic alteration of spinal cord dorsal horn neurons and neuropathic pain in the rat. Pain, 72:355-366, 1997. Mao, J., Price, D.D., Lu, J. and Mayer, D.J.: Antinociceptive tolerance to the mu-opioid agonist DAMGO is dose-dependently reduced by MK-801. Neurosci. Lett., 250:193-196, 1998. Mao, J.: NMDA and opioid receptors: their interactions in antinociception, tolerance, and neuroplasticity, Brain Res. Rev. 30:289-304, 1999. Mayer, D.J., Mao J., Holt, J. and Price, D.D.: Cellular mechanisms of neuropathic pain, morphine tolerance, and their interactions, Proc. Natl. Acad. Sci. USA, 96: 7731-7736, 1999. Mao, J., Sung B., Ji, R.R. and Lim, G.: Neuronal apoptosis associated with morphine tolerance: evidence for an opioid-induced neurotoxic mechanism. J. Neurosci., 22:7650-7661, 2002. Mao, J., Sung, B., Ji, R.R. and Lim, G.: Chronic morphine induces downregulation of spinal glutamate transporters: implications in morphine tolerance and abnormal pain sensitivity. J. Neurosci., 22:8312-8323, 2002. Sung, B., Lim, G. and Mao, J.: Altered expression and uptake activity of spinal glutamate transporters following nerve injury contributes to the pathogenesis of neuropathic pain in rats, J Neurosci, 23;2899-2910, 2003. Lim, G., Sung, B., Ji, R.R. and Mao, J.: Upregulation of spinal cannabinoid-1-receptors following peripheral nerve injury enhances the effects of Win 55,212-2 on neuropathic pain behaviors in rats, Pain, 105:275-283, 2003. Ballantyne, J. and Mao, J.: Opioid therapy for chronic pain. New Eng J Med, 349:1943-1953, 2003. Cohen, S., Chang, A.S., Larkin, T. and Mao, J.: The IV Ketamine Test: A predictive response tool for oral dextromethorphan treatment in neuropathic pain. Anesth Analg, 99:1753-1759, 2004. Wang, S., Lim, G., Zeng, Q., Yang, L., Sung, B., Mao, J.: Expression of spinal glucocorticoid receptors following peripheral nerve injury contributes to neuropathic pain behaviors in rats. J Neurosci, 24:8595-8605, 2004. Lim, G., Wang, S., Zeng, Q., Sung, B. and Mao, J.: Evidence for a long-term influence of chronic morphine on morphine tolerance: role of central glucocorticoid receptors. Pain, 114:81-92, 2005. Wang, S., Lim, G., Zeng, Q., Yang, L., Sung, B. and Mao, J.: Central glucocorticoid receptors modulate the expression and function of spinal NMDA receptors after peripheral nerve injury. J Neurosci, 25:488-495, 2005. Lim, G., Wang, S., Zeng, Q., Sung, B. and Mao, J.: Central glucocorticoid receptors contribute to the mechanisms of morphine tolerance. Anesthesiology 102:832-837, 2005. Lim, G., Wang, S., Zeng, Q., Sung, B., Yang, L. and Mao, J.: Expression of spinal NMDA receptor and PKC after chronic morphine is regulated by spinal glucocorticoid receptor. J. Neurosci. 25:11145-11154, 2005 Wang S, Lim G, Yang L, Sung B, Mao J: Downregulation of spinal glutamate transporter EAAC1 following nerve injury is regulated by central glucocorticoid receptors in rats. Pain, 120:78-85, 2006. Cohen S, Larkin T, Mao J. The IV ketamine test predicts subsequent response to an oral dextromethorphan treatment regimen in fibromyalgia patients – A clinical trial. J Pain, 7:391-398, 2006. Lim J, Lim G, Sung B, Wang S, Mao J. Intrathecal midazolam regulates spinal AMPA receptor expression and function after nerve injury in rats. Brain Res, 1123:80-88, 2006. Wang S, Yao Z, Wang J, Ai Y, Zhang Y, Gu H, Li D, Mao J. Evidence for a distinct group of nestinimmunopositive neurons within the basal forebrain of adult rats. Neuroscience, 142:1209-1219, 2006. Chang G., Chen, L. Mao J. Role of opioid tolerance and opioid-induced pain sensitivity in clinical opioid use. Med Clin North America 91:199-211, 2007. Cohen S. Larkin T. Mao J. Postamputation pain from Operation Iraqi Freedom. J Trauma, 62:759-761, 2007. Sung B, Wang S, Zhou B, Lim G, Yang L, Zeng Q, Lim JA, Mao J. Altered spinal arachidonic acid turnover after peripheral nerve injury regulates regional glutamate homeostasis and neuropathic pain behaviors in rats. Pain, 131:121-131, 2007. Wang S, Lim G, Mao J, Sung B, Yang L, Mao J. Central glucocorticoid receptors regulate the upregulation of spinal cannabinoid receptors after peripheral nerve injury in rats. Pain, 131:96-105, 2007. Singla A, Chen L, Stojanovic M, Mao J. Differential diagnosis of hyperalgesia, toxicity, and withdrawal from intrathecal morphine infusion. Anesth & Analg, 2007, 105:1816-1819. Gu X, Wang S, Yang L, Sung B, Lim G, Zeng Q, Chang Y, Mao J. Time-dependent effect of epidural steroid on pain behavior induced by chronic compression of dorsal root ganglion in rats. Brain Res 2007, 1174:39-46. Gu X, Yang L, Wang S, Sung B, Lim G, Zeng Q, Chang Y, Mao J. A rat model of radicular pain induced by chronic compression of dorsal root ganglion in rats. Anesthesiology 2008, 108:113-121. Cohen SP, Christo PJ, Wang S, Chen L, Stojanovic MP, Shields CH, Brummett C, Mao J. The Effect of opioid dose and treatment duration on the perception of a painful standardized clinical stimulus. Reg Anesth Pain Med 2008, 33:199-206. Zeng Q, Wang S, Lim G, Yang L, Mao J, Sung B, Chang Y, Lim JA, Guo GS, Mao J. Exacerbated mechanical allodynia in rats with depression-like behavior. Brain Res 2008, 1200:27-38. Cohen SP, Wang S, Chen L, Kurihara C, McKnight G, Marcuson M, Mao J. An intravenous ketamine test as a predictive response tool in opioid-tolerant patients. J Pain Symp Manage 2009; [Epub ahead of print] Vorobeychik Y, Chen L, Bush MC, Mao J. Improved opioid analgesic effect after opioid dose reduction. Pain Medicine 2008; 9:724-727. Yang L, Wang S, Sung B, Lim G, Mao J. Morphine induces ubiquitin-proteasome activity and glutamate transporter degradation. J Biol Chem 2008; 283:21703-21713. Lim G, Wang S, Zhang Y, Tian YH, Mao J. Spinal leptin contributes to the pathogenesis of neuropathic pain. J Clinical Investigation, 2009; 119:295-304. Chen L, Malarick C, Seefeld L, Wang S, Houghton M, Mao J. Altered quantitative testing outcome in subjects with chronic opioids. Pain, 2009; 143:65-70. Wang S, Zhang L, Lim G, Sung B, Tian Y, Chou CW, Hernstadt H, Rusanescu G, Mao J. A combined effect of dextromethophan and melatonin on neuropathic pain behavior in rats. Brain Res, 2009; Accepted. Hernstadt H, Wang S, Lim G, Mao J. Spinal translocator protein (TSPO) modulates nociceptive behavior in rats with CFA-induced monoarthritis. Brain Res, 2009; Accepted. Wang S, Lim G, Mao J, Sung B, Mao J. Regulation of the trigeminal NR1 subunit expression following temporomandibular joint inflammation in rats. Pain, 2009; 141:97-103. C. Research Support. List selected ongoing or completed (during the last three years) research projects (federal and non-federal support). Begin with the projects that are most relevant to the research proposed in this application. Briefly indicate the overall goals of the projects and responsibilities of principal investigator identified above. ONGOING 1. "Glutamate Uptake and the Pathogenesis of Neuropathic Pain" Principal Investigator: Jianren Mao, M.D., Ph.D. Agency: NIH-NINDS; Type: RO1 NS45681 Period: 12/1/2003 – 11/30/2009 (no-cost extension) The long-term objective of this project is to study the role of spinal glutamate transporters in the mechanisms of neuropathic pain. 2. “Opioid-induced Pain Sensitivity: Clinical Diagnosis and Management” Principal Investigator: Jianren Mao, M.D., Ph.D. Agency: NIH-NIDA; Type: RO1 DA022576 Period: 9/30/2006 – 4/30/2011 This is a clinical research project. The goal is to explore clinical features of opioid-induced pain sensitivity using quantitative sensory testing. 3. “Comorbidity between Depression and Orofacial Pain” Principal Investigator: Jianren Mao, M.D., Ph.D. Agency: NIH-NIDCR; Type: RO1 DE018538 Period: 7/1/2007 – 6/30/2012 The long-term goal of this project is to examine the relationship between depression and orofacial pain utilizing a combined rat model of depression-like behavior and orofacial pain behavior. 4. “Cellular Mechanisms of Orofacial Pain” Principal Investigator: Jianren Mao, M.D., Ph.D. Agency: NIH-NIDCR; Type: RO1 DE018214 Period: 8/4/2008 – 7/31/2013 The goal is to investigate the trigeminal mechanisms of orofacial pain, particularly the role of glial-neuronal interactions in the mechanisms of orofacial pain. 5. “Translational Research on Prescription Drug Abuse” Principal Investigator: Jianren Mao, M.D., Ph.D. Agency: NIH-NIDA; Type: P20 DA026002 Period: 9/15/2008 – 6/30/2012 The goal of this center project is to study the neurobiology of prescription drug abuse in human subjects as well as in animal models. COMPLETED 1. "Cellular Mechanisms of Hyperalgesia and Opioid Tolerance" Principal Investigator: Jianren Mao, M.D., Ph.D. Agency: NIH-NIDA; Type: RO1 DA08835 Period: 4/1/1995 – 4/30/2007 2. "Mechanisms of Neuropathic Pain Modulation by Cannabinoids" Principal Investigator: Jianren Mao, M.D., Ph.D. Agency: NIH-NINDS; Type: RO1 NS42661 Period: 4/30/2003 – 5/31/2008