Physical Chemical Change Lab
advertisement

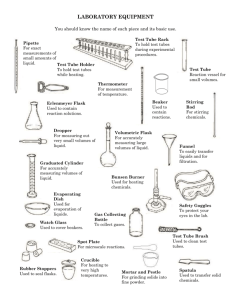
Lab: Chemical and Physical Changes Name: Date: A. Purpose: To investigate the criteria used to distinguish between physical and chemical changes in matter. B. Background: Have you ever thought of your eyes as powerful tools for studying chemistry? Many of the properties of matter and the changes it undergoes can easily be determined through careful observation. Physical properties include color, odor, density, solubility, and the state of the matter. Chemical properties describe the changes that take place when new substances are formed during a chemical reaction. When matter undergoes a change, it is classified as either a physical change or chemical change. During a physical change, only the size, temperature, or physical state of the substance changes. Melting, dissolving, grinding, and evaporating are all physical changes. No new substances are produced during a physical change. However, chemical changes always result in the formation of one or more new substances. The rusting of iron, during which the new substance iron(III) oxide forms from iron (Fe) and oxygen (O2), is an example of a chemical change. Evidences of chemical changes are : a change of state (a gas or precipitate is produced), a change in color, a change in odor, or a change in temperature. The Law of Conservation of Mass states that in any chemical or physical change mass is conserved (does not change). You will record the mass of the test tubes before and after combining them to see if this law holds true for these reactions. In this experiment you will observe a variety of materials and describe their physical properties. You will then cause some of the substances to undergo changes. Based upon your observations, you will determine whether the changes are physical changes or chemical changes. C. Materials: 12 medium test tubes Test tube rack Stir rod Filter paper Funnel Electronic balance Sulfuric Acid (12M H2SO4) Teacher only handles Test tube holder 0.1 M hydrochloric acid (1.0 M HCl) Solid sodium carbonate (Na2CO3) Solid calcium nitrate (Ca(NO3)2 Magnesium ribbon Sand Sodium chloride D. Procedure: 1. Obtain two medium sized test tubes. Place a small amount (enough to fill 1/4 inch of the scoopula tip) of solid sodium carbonate in to test tube 1. Fill the test tube about a third full with distilled water and stir with the glass stir rod. Record two or three physical properties of sodium carbonate in Table 1.1. 2. Repeat step one with calcium nitrate, placing it into test tube 2. BE SURE TO RINSE AND DRY STIR ROD BETWEEN SOLUTIONS TO PREVENT CONTAMINATION. 3. Determine and record the mass of the combined test tubes in Table 1.2. Place a small beaker on the balance and press the “Tare” button. The scale should read 0.00 g. Then add the two test tubes and record in Table 1.2. 4. Note the temperature by feel of the two test tubes. Combine the contents of test tubes one and two and observe. Record your observations in table 1.3. If no chemical change occurs, indicate by writing “Physical change only.” Again note the temperature (Be careful, some reactions can cause the test tubes to get very hot). 5. Determine the mass of test tubes 1 and 2 and their contents after combining them and record in Table 1.2. 6. Clean and rinse the test tubes (Contents of the test tubes can go in the sink). 7. Repeat steps 1-6 using only distilled water in test tube 3 and dry sodium chloride (NaCl) in test tube 4 (Do not add water to test tube 4 before combining test tube 3 and test tube 4). 8. Repeat steps 1-6 using only distilled water in test tube 5 and dry sand in test tube 6 (Do not add water to test tube 6 before combining test tube 5 and test tube 6). 10. Repeat steps 1-6 using 1.0 M HCl in test tube 7 (already a solution, so no need to add more water) and sodium carbonate in test tube 8. 11. Repeat steps 1-6 using 1.0 M HCl in test tube 9 and add a small piece (2-5 cm) of magnesium ribbon to test tube 10( do not add water to test tube 6). 12. Fill a test tube 1/3 full with sugar, bring over to teacher to have Sulfuric acid added. Be sure to hold test tube with test tube holder, teacher will demonstrate. Record results. Be sure to set tube in proper disposal container!! Table 1.1 Physical Properties Test tube Contents Na2CO3 1 Distilled water Ca(NO3)2 2 Distilled water 3 Distilled water 4 Solid NaCl 5 Distilled water 6 Dry sand Physical properties 7 1.0 M HCl 8 Na2CO3 Distilled water 9 01.0 M HCl 10 Magnesium ribbon (solid) 11 Dry sugar Table 1.2 Masses of substances before and after combined. Test tubes Mass of combined Mass of test tubes after test tubes before combining combining. 1 and 2 3 and 4 5 and 6 7 and 8 9 and 10 11 (before acid) (after acid) Change in mass Table 1.3 Chemical properties of substances. Test tubes Evidence of chemical change (if present) 1 and 2 3 and 4 5 and 6 3,4,5 and 6 7 and 8 9 and 10 11 12 E. Analyses and Conclusion: 1. Explain how to distinguish a physical property from a chemical property. 2. Explain how to distinguish a physical change from a chemical change. 3. List five indications of a chemical change. 4. List and describe the physical changes in the above combinations of test tubes and/or single test tubes. 5. Which test tube combination(s) showed the formation of a precipitate (solid)? 6. Which test tube combination(s) showed the formation of a gas? 7. Which test tube combination(s) showed a temperature change? 8. Which test tube combination(s) showed a change in color? 9. State the law of conservation of mass. 10. Which, if any, of the above chemical reactions showed a change in mass? Explain.