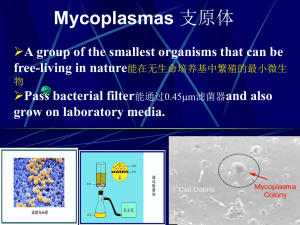
biochemical and serological identification of mycoplasmas from
advertisement

ISRAEL JOURNAL OF VETERINARY MEDICINE Vol. 58 (2-3) 2003 BIOCHEMICAL AND SEROLOGICAL IDENTIFICATION OF MYCOPLASMAS ISOLATED FROM PNEUMONIC BOVINE LUNGS IN NIGERIA A.T.P. Ajuwape1, J.O. Ikheloa1, M.O. Ojo1, O.O. Alaka2 and A.I. Adetosoye1 1. Department of Veterinary Microbiology and Parasitology 2. Department of Veterinary Pathology, University of Ibadan; Ibadan. Nigeria. Abstract Thirty-nine strains of Mycoplasma were isolated from pneumonic lung tissues of cattle observed at the abattoirs. The strains were biochemically characterized and identified serologically. On the basis of biochemical characterization the strains were divided into 3 groups. [A] Twenty-two strains which hydrolyzed glucose, digested serum and reduced tetrazolium chloride. [B] Eight strains which catabolized arginine only. [C] Nine strains which reduced tetrazolium chloride and had phosphatase activity. Group A strains were identified serologically as M. mycoides subsp. Mycoides; group B strains were identified serologically as M. arginini while the strains in group C were identified as M. bovis. The lung tissue showed acute fibrino-purulent pleuropneumonia. Isolation of M. bovis showed that this organism plays an important role is severe pneumonia. Efforts should be made to produce a vaccine against M. bovis, similar to that for M. mycoides subsp. Mycoides, to protect calves against severe pneumonia. Introduction Mycoplasma mycoides subsp mycoides is the causative agent of contagious bovine pleuropnuemonia[CBPP]; a highly contagious disease and the most important respiratory disease in the world [4]; as well as a serious economic disease of cattle in many parts of the world including tropical Africa, France, India, Italy, Middle East, Portugal and Spain [18,20]. Contagious bovine pleuropneumonia has caused greater losses in cattle than any other disease after Rinderpest [15]. This disease was eradicated from the U. S. A. in 1892 [1]; from South Africa by launching the JP28 campaign in 1971, but the disease has been on the increase since 1986 [12]. In Nigeria CBPP outbreaks occurred in Kano, Katsina, Borno, Sokoto and Kaduna States [14]. The re- emergence of the disease in Nigeria might have been due to the introduction of chronic carriers from neighbouring African countries such as Cameroon, Chad and Niger Republic. It has been reported that outbreaks occurred when healthy and carrier animals gather at river banks during the dry season [16]. This close contact between the healthy and carrier animals facilitated the transmission of Mycoplasma responsible for the disease. This investigation was undertaken to identify Mycoplasma species associated with pneumonia in cattle in Nigeria. Materials and Methods During the rainy season, from May to September 1997, pneumonic lung tissues and blood samples were collected from cattle with pneumonia at abattoirs in Maiduguri, Kaduna, Sokoto and Ibadan. The clotted blood was centrifuged and the sera were stored on ice. Each pneumonic lung was cut into two, one half was put into 10% formalin while the remaining half was put into a sterile nylon bag, frozen and transported on ice to Ibadan and kept at -200C at the Department of Veterinary Microbiology and Parasitology, University of Ibadan. Bacteriology The tissues were seeded on blood agar, MacConkey agar and Medium N Mycoplasma agar [9] containing no glucose, according to standard methods (2,10) for the isolation of pathogenic bacteria and Mycoplasma. Characterization of Mycoplasma isolates: The Mycoplasma isolates were respectively cloned 3 times, and an isolated colony was inoculated in Mycoplasma broth and incubated at 370C for three days after which the culture was inoculated on Mycoplasma agar. This procedure was repeated three times. Absence of bacterial reversion The Mycoplasma isolates were subcultured in Medium N [Mycoplasma agar without penicillin]. Gaseous requirement All the Mycoplasma isolates were grown in Mycoplasma medium N. 0.01 ml of 10 fold serial dilution from 10-6 was inoculated on blood agar and incubated aerobically. They were also inoculated on Mycoplasma agar and incubated at 370C in a candle jar. The plates were examined every 48 hours to record Mycoplasma growth. The plates were inoculated for 10 days and plates not showing colonies resembling Mycoplasma were discarded. Morphological studies The colonial morphology was studied with a stereomicroscope. All the strains were grown anaerobically in a candle jar for 10 days. Each colony was examined for morphological characteristics of Mycoplasma. Sensitivity to digitonin The tests were performed as the disc growth inhibition test[5]. Filter paper discs [6.35mm in diameter] were soaked with 0.02ml of 1.5% [W/V] ethanolic solution of digitonin [Sigma Chemical Co. St. Louis, U.S.A] and dried overnight at 370C. Seven milliliter of medium N (Mycoplsma agar) without glucose was dispensed in 5 cm glass petri dishes. The plates were dried before use and inoculated with 0.01ml of culture containing 1x105 CFU per ml. The running drop technique was employed. The discs were pressed gently on the middle of the inoculated area. The plates were incubated at 370C in a candle jar. The inhibition zone was measured after 5 days of incubation at 370C. Tests for cholesterol requirement This was carried out as described previously (7). The inoculated plates were examined for growth every second day during incubation at 370C for 10 days. The requirement for cholesterol was determined indirectly by a sensitivity test against digitonin by the method described elsewhere [11] Tetrazolium reduction: The test was performed by incorporating 5ml of 2,3,5, triphenyl tetrazolium hydrochloride [2% solution W/V] to 15ml of medium N containing no glucose in vacutainer tubes. The pH of the medium was adjusted to 7.5. Phosphatase activity The test was performed by inoculating medium N with the test organism. The medium N contained heated horse serum plus 200,000 I.U/ml penicillin, (0.20ml), 1.0ml of thallium acetate and 1ml of phenolphthalein sodium diphosphate. The pH was adjusted to 7.8. The organisms under test were incubated for 4 days, after which a drop of 30-40% potassium hydroxide was added. A positive reaction was shown by a pink colour, which faded very quickly. Test for proteolytic activity Strands of developed black and white film, measuring 90x5mm were used. The film was sterilized by autoclaving as 1000C for 1 hr. The sterilized films was dropped into Medium N culture of the Mycoplasma isolate under test. During the period of incubation the gelatin on the film was hydrolysed. A positive test showed black granules of gelatin at the bottom of the test tube, which was incubated for 7 days. Glucose hydrolysis The medium and the test substrates were prepared as described previously [10]. The test substrates were made by adding 1.0ml of 50% [w/v] stock solution of glucose to the broth medium. The medium was inoculated with a single colony. The reaction was read by comparison with inoculated and uninoculated base medium as well as with uninoculated test substrate. A positive result was recorded when a colour change (red-yellow) between the inoculated test substrate and each of the control substrates was present. For negative reaction there was no colour change. The test was observed for 14 days. Catabolism of Arginine The base medium was the same as for glucose fermentation. The pH was adjusted to 7.3. The test substrate consisted of base medium with the addition of 4.25ml L-arginine[Sigma] of 30% (w/v) stock solution. Formation of Film and Spots Medium N was prepared with a high (40ml) concentration of the horse serum. The plates were inoculated and incubated at 370C for 14 days. They were examined for film and spots after 3,7 and 14 days incubation. Growth inhibition test The test was carried out by the running drop technique. Medium N [9] was prepared. The agar plated was inoculated with 0.01ml of test containing 105 and 106†CFU/ml. After drying, a 6mm disc soaked with antiserum was gently placed in the middle of the Mycoplasma streak. The plate was incubated at 370C for arginine-positive strains while others were incubated at a lower temperature (room temperature) in an anaerobic, candle jar. Histopathology Lung tissues were fixed in 10% phosphate buffered formalin dehydrated in graded dilutions of ethanol and embedded in paraffin. Thin sections (5µ) of tissues obtained on the grease-free glass slides were stained with heamotoxylin and eosin. Stained sections were examined for histological changes. Results Morphological studies:- colonies of Mycoplasma strains after incubation at 370C for four days exhibited the characteristic fried egg appearance with an elevated central spot. Reversion experiment No bacteria-like colonies developed on the media without penicillin during passage of the strains. Cholesterol requirement All strains were digitonin sensitive. Gaseous requirement They all grew well aerobically under candle jar. Growth at 370C: All the strains grew well at 370C. Biochemical characteristics The strains were biochemically studied using glucose, arginine, phosphate, 2, 3, 5-tetrazolium chloride, protein digestion. Table I shows the biochemical reactions of the isolates. Table 1. Biochemical characteristics of bovine mycoplasmas. No. of strains Sensitivity to digitonin (mm) Catabolism of Reduction of arginine tetrazolium chloride Phosphatase activity Digestion of serum Film & Sp M.mycoides 22 4-5 - + - + - M.arginini 8 3-4 + - - - - M.bovis 9 4.5 - 6 - + + - - Growth inhibition test The growth of 22 Mycoplasma strains which fermented glucose, was inhibited by the antiserum to M. mycoides subsp. Mycoides. The growth of 8 Mycoplasma strains was inhibited by antiserum to M. arginni while the growth of 9 Mycoplasma strains were inhibited by antiserum to M. bovis. Film and Spots; None of the Mycoplasma strains showed film or spots. Histopathology The pneumonic lung tissues showed typical acute fibrinopurulent pleuropneumonia consistent with those associated with early stages of contagious bovine pleuropnuemonia. Figure I shows severe congestion, oedema and fibrin exudation into thickened interalveolar spaces, subpleural space and into alveolar lumen. Some alveoli were confluent and emphysematous, while a few were collapsed. Numerous neutrophils, macrophages, many of which have haemosiderin pigments and engulfed erythrocytes, and epithelioid cells were found in distended interalveolar septa and around blood vessels. Bronchiolar epithelium were hyperplastic and folded and considerable quantity of oedematous fluid and inflammatory cells were found in the lumen. No pathogenic bacteria other than Mycoplasma organisms, of any clinical significance, were isolated from these lung tissues. Figure 1b, shows lung tissue with large quantities of fibrin in interlobular spaces. H & E x 100. Figure 1a, shows histopathological c tissue with severe pulmonary oedema and with infiltration of neutrophils H & E x 100 Figure 1c, shows lung tissue with neutroph spaces H & E x 100. Table 2. Summary of growth inhibition tests on mycoplasma strains grouped by biochemical characterization. Species group Antisera M. mycoides subsp.mycoides M. arginini M. bovis M.mycoides subsp mycoides A 22 - - M. arginini B - 8 - M. bovis - - 9 Discussion In this study, Mycoplasma mycoides subsp mycoides, M. bovis and M. arginini were isolated from the pneumonic lung tissues of cattle slaughtered at the abattoirs. In spite of the JP 28 campaign of 1971 to eradicate and control CBPP in Africa and the annual vaccination of cattle against CBPP in Nigeria, especially in the Northern States where about 90% of Nigerian cattle live, CBPP, a deadly and the most important respiratory disease of cattle, is still present in Nigeria. M.mycoides subsp. mycoides were isolated from pneumonic lungs at slaughter in Sokoto and Kaduna abattoirs. The prescence of CBPP in these States and the recovery of this species of Mycoplasma from pneumonic cattle might have been due to the introduction of carrier animals into Nigeria from the neighbouring countries of Niger, Chad and Cameroon. The animals may have been missed during vaccination or they may have been vaccinated but due to poor storage of the vaccine, the vaccinated animal might not have been protected. In the study M. bovis was isolated from pneumonic lung tissues of cattle observered at the abattoirs in Maiduguri. Similar finding was obtained recently [8]. At that time M. bovis, M. dispar, M. ovipneumoniae and Acholeplasma laidlawi were isolated from lung tissues collected at Maiduguri abattoirs. In this investigation 31% of the sera collected were positive for M. bovis. Reports from other parts of the world (3,12,15,19) indicated that M. bovis is an important pathogenic agent responsible for severe pneumonia in cattle. Consequent to the isolation of M. mycoides subsp mycoides and M. bovis in this investigation, it is suggested that efforts should be made to ensure that cattle are vaccinated regularly against Mycoplasma species causing pneumonia from the species of Mycoplasma responsible for the disease in such areas. The vaccine should be used along with TI-44 vaccine. Movement of animals from neighbouring countries should be properly controlled. Government agencies should ensure that animals crossing Nigerian borders are vaccinated and free of CBPP to prevent the introduction of carriers into the herd. Acknowledgement This work was financed by the National Agricultural Research Project RGS 024 and Prof. A.I. Adetosoye. Department of Veterinary Microbiology and Parasitology University of Ibadan; Ibadan. Nigeria. LINKS TO OTHER ARTICLES IN THIS ISSUE References 1. 1. Animal Health Year Book 1980 2. Barrow, G.J. and Feltham, R.K.A.: Cowan and Steel`s manual for identification of medical bacteria 3rd edition. Cambridge University Press. 1993. 3. Binder, A.G., Amtsberg, Dose, S, Fisher, W., Scholtz, H. and Kirchoff, A.: Isolation of Mycoplasma and bacteria from cattle with respiratory disease. J. Vet. Med. Series B: 430-435, 1990. 4. Cassel, G.H, Clyde Jr., W.A., David, J.K.: Mycoplasma IV. S. Razin and M.F. Barile [eds] Academic press inc. Orlando. pp 65-106, 1985. 5. Clyde, W.A.: Mycoplasma species identification based upon growth inhibition by specific antisera. J. Immunol, 92: 958-965, 1964. 6. Curasson, G.: Traite de pathologic exotique Veterinaire et compare temo 1, pp 590-606 Vigot Freres, Paris. 7. Edward, D.G.: Determination of sterol requirement for mycoplasmatales. J. General Microbiol. 69: 205-210, 1971. 8. Egwu, G.O., Ameh, J.A. and Aliu, M.M.: Detection of antibodies to Mycoplasma bovis in Nigeria cattle by Enzyme Linked Immunosorbent Assay [ELISA]. Trop. Vet. 14: 163-167,1996. 9. ErnÔ, H. and Stipkovits, L.: Bovine mycoplasmas: Cultural and biochemical studies I. Acta Vet. Scand. 14a: 436-449, 1973. 10. ErnÔ, H. and Stipkovits, L.: Bovine mycoplasmas.: Cultural and biochemical studies II. Acta Vet. Scand. 14b: 450-463, 1973. 11. Freundt, E. A., ErnÔ, H., Kunze, M. and Black, F.T.: The sensitivity of mycoplasmatales to sodium-polyanethol sulfonate and digitonin Zbl. Bakt. Parasit. Infekt Hyg, Abt, 225: 104-112, 1973 12. Kim, J.Y., Cho, S.K. and Park, J.M.: Studies on Mycoplasma pneumonia of cattle Research Report of the Rural Development Administration Veterinary 31: 30-35, 1989. 13. Mohammed, L.U.: Economic Evaluation of CBPP in Nigeria. N.V.M.A. Conf. Jos 24-26 Nov [1986] pp 2-4. 14. Nawatte, D.R.: Resurgence of CBPP in Nigeria. Res. Sci. Tech. Intern Epiz 13: 799-804, 1991. 15. Nicolet, J.:; Mycoplasma bovis spread of a new pathogen in the cattle population in Switzerland. Schwiz. Archiv Für. Tierheilkunde 136: 81-82, 1994 16. Nwanta, J.N. and Umoh, J.U.: Epidemiology of contagious bovine pleuropneumonia [CBPP] in the Nothern States of Nigeria an update Revue-d-Elevage et de Medicine Veterinaire des pays Tropicaux 45: 17-20, 1992. 17. O.I.E. Meeting of the FMD and other Epizootics commission OIE Paris. Jan. 11- 20, 1995. 18. Provost, A.P., Perreau, A., Bread, C. Je Goff, Martel, J.L. and. Cottew, G.S.: Contagious bovine pleuropnuemonia. Rev. Sci. Tech. Intern Epiz 6: 625-679, 1987. 19. Reilly, G.A.C., Ball, H.J., Cassidy, J.P. and Bryson,.T.D.G.: First reported isolation of Mycoplasma bovis from pneumonic calves in Northern Island. Vet. Rec. 133: 550-551, 1993. 20. Tek laak, E.A; Contagious bovine pleuropneumonia. Vet. Quart. 15: 110-104, 1992. Acknowledgement This work was financed by the National Agricultural Research Project RGS 024 and Prof. A.I. Adetosoye. Department of Veterinary Microbiology and Parasitology University of Ibadan; Ibadan. Nigeria.