Systematic Review/Meta-analysis
advertisement

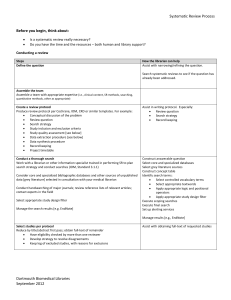
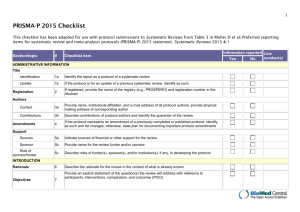
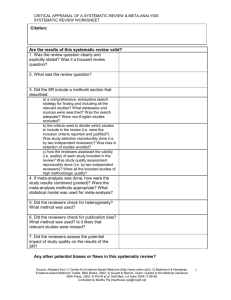
1 of 3 UNIVERSITY OF COLORADO HOSPITAL SYSTEMATIC REVIEW OR META-ANALYSIS CRITIQUE Facilitator of Journal Club Name: Date: Systematic Review: It is a summary of evidence on a particular topic, conducted using a rigorous process for identifying, appraising, and synthesizing numerous studies to answer a clinical question. Meta-analysis: A systematic review is the quantitative synthesis of multiple studies. A meta-analysis produces a summary statistic that represents the effect of the intervention across multiple studies. Both systematic reviews and meta-analyses yield the strongest level of evidence (LOE I) to use when making practice decisions. Article Reviewed: (Author names, Title of article, Journal, Year, Volume, Page numbers) (Facilitator Info: Before you being your critique, read the critique form to be aware of what questions you should answer after reading the article. Then, read the article for a first overview or impression. Finally, read the article again, slowly. Stop and answer the questions from the critique form. Highlight the answers in the article. Also, on the critique form, write page numbers of where answers can be found to help you when you are leading the discussion at the Journal Club. As you plan for the journal club, refer to the Research and Evidence-Based Practice Manual 2nd Edition. Chapter 7 titled Participating in a Journal Club provides step by step advice for the process.) Article Purpose: What are the review questions? Are they clearly and explicitly stated? Evaluation of Validity of the Review: How was the clinical question focused with regard to: o The population? o The intervention? o The outcome measure? Was a systematic approach used for the literature search: o What search terms were used? o What years were included? o What databases were used? o Were all the relevant databases, and appropriate search terms used for the search? o Were other searches performed to enhance the database retrieval (i.e. reference lists, additional bibliographies, or professional organizations)? What criteria were used to select studies to be included in the review? o Were the criteria established before the review began? 2 of 3 Were inclusion criteria based upon: o Sample characteristics? o Interventions studied? o Research methods? o Research Design? o Outcomes examined? Is there a table of the studies included in the review with a brief synopsis of each study? Were the methods used to critique the studies described? Were the critiques carried out by more than one person? Interpreting the Results: Were the results consistent across studies? What were the overall results of the review? How large is the intervention or treatment effect (for meta-analysis only)? How precise is the intervention or treatment (use Confidence Intervals to assess)? Are the conclusions or summary supported by the reported data? Applicability of Results to Practice What are the clinical implications for my clinical practice? Are my patients similar to the patients included in the original studies? Is the intervention feasible in my setting? o What other information would I need to assess this? Were all important outcomes considered? o What important outcomes were not considered? 3 of 3 Do the benefits outweigh the potential harm/risk? What is my clinical assessment of the patient or practice setting and are there any contraindications or circumstances that would inhibit me from implementing the treatment/intervention? How do my patient’s (and family) preferences fit with the intervention being considered? What is the level of evidence for this study? Strongest Evidence Level 1 2 One or more randomized controlled trials 4 Controlled trial (no randomization); quasi-experimental Case-control or cohort study 5 Weakest Evidence Evidence Systematic review & meta-analysis of randomized controlled trials; clinical guidelines based on systematic reviews or meta-analyses 6 7 8 Systematic review of descriptive studies; systematic review of qualitative studies (metasynthesis) Single descriptive or qualitative study Program evaluation, research utilization, quality improvement projects; case reports (JC sentinel event); benchmarking studies (NDNQI data, UHC reports); or research-based clinical practice guidelines Expert opinion (authorities and/or reports of expert committees) textbooks, clinical product guidelines; non-research based clinical practice guidelines