Amine Chemistry - Chemistry Courses: About
advertisement

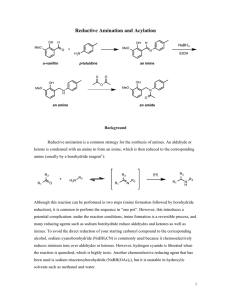
Green Amine Chemistry Read: Mohrig, Technique 2—Green Chemistry US EPA website(www.epa.gov)—Green Chemistry Purpose: Perform a one-pot, multistep synthesis that demonstrates green chemistry Background: Amines are an important functional group in organic chemistry. Although they primarily serve as nucleophiles and bases, their reactions are quite diverse. In this experiment, we will be conducting a one-pot, three-step reaction of amines, outlined in Figure 1. Figure 1. 1. CH3 H CH3 OH O H H2N H3CO N OH 2. NaBH4/ethanol 3. acetic anhydride H3C O OCH3 In a one-pot reaction, distinct reaction steps are carried out without purification or isolation of the intermediate. This is convenient because it saves time and can lead to a higher overall yield of final product. This synthesis is also considered to be a relatively “green” synthesis. There is an ongoing push in chemistry to develop environmentally friendly reactions. This reaction is green in a number of ways. First, the reactions are all solvent-less or use fairly nontoxic solvents such as ethanol. Second, it is high-yielding, meaning that there is not a lot of unreacted starting material. Also, it is atom-efficient. The first reaction has water as its only side product. In this experiment, you will be carrying out three distinct reactions all using the chemistry of the amine functional group. The sequence starts with p-toluidine (an amine) reacting with o-vanillin, an aldehyde. Normally this reaction is catalyzed by acid, but in this case, the phenol acts as an internal acid catalyst. The final product of step one is a zwitterion, meaning that it is positively and negatively charges simultaneously. This can happen when the same molecule contains an acid and a base. The second step is a reduction by sodium borohydride. In the third step, acetic anhydride reacts by acylating the most nucleophilic atom of Intermediate B. The amide that is formed is quite stable and can be easily isolated and purified. In this experiment, you will conduct all three reactions in sequence. This will be a more qualitative lab. You don’t have to isolate or characterize the intermediates, but record observations of each reaction. You will obtain a final yield of the unpurified product. You will the purify a small amount of the crude product and obtain a mp. Pre lab questions: 1. Based on your knowledge of amine chemistry, predict the structures of Intermediates A and B. (Hint: Step one has a phenol proton, which is quite acidic.) Figure 2. H CH3 H OH O H3CO N OH H3C OCH3 O CH3 1. 3. acetic anhydride H2N 2. NaBH4 Intermediate B Intermediate A 2. Fill out a stoichiometry table for this set of reactions. List each reagent as either catalytic, limiting, or excess. Fill in the “Product” column with the theoretical amounts to be produced. Reagent MW Moles Grams Density Volume Limiting? o-vanillin p-toluidine NaBH4 0.760 g .535 g 0.1 g Acetic anhydride Product 2.0 mL (Hint: Why is sodium borohydride not the limiting reagent even though a smaller number of moles of this reagent used? Consider the number of hydride equivalents in sodium borohydride.) Procedure: (Step 1) Pre-weigh a 205 mL beaker with a stir rod. Place exactly 0.760 g of ovanillin on one side of th pre-weighed 250 mL beaker with stir rod and 0.535 g of ptoluidine on the other side of the beaker. (Caution: organic amines are often carcinogens) Use the glass stir rod to gently mix a portion of the two compounds together and observe the reaction. Stir and grind the two compounds together thoroughly until you obtain a homogenous dry powder, Intermediate A. (It will initially be wet, but will then dry.) Weigh the beaker, stir rod, and product after the reaction is complete. (Step 2) Dissolve Intermediate A in about 15 mL of 95% ethanol and add a stirbar. Stir to partially dissolve Intermediate A. Add 0.1 g of sodium borohydride in small portions with stirring. Within about ten minutes, the solid should be completely dissolved. Make observations. (Step 3) Add 2 mL of glacial acetic acid to the reaction mixture slowly. (The acetic acid serves to destroy excess borohydride and neutralize the phenoxide ion.) Add 2 mL of acetic anhydride and warm the solution on a stir plate for 5-10 minutes. Move the solution to a cool stirplate and allow the reaction to go to room temperature. Stir the solution as rapidly as possible and slowly and continuously pour in 75 mL of water. Continue to stir until the amide precipitates. Cool the reaction in an ice bath and collect the solid by filtration. Air dry for 5-10 minutes. Obtain a final mass of the slightly wet compound. Recrystallize a 0.2 g sample of your final product from hexanes. It takes a relatively large amount of hexanes to recrystallize, so use at least a 100 mL beaker. Obtain a melting point of the recrystallized compound. Results and observations: Write observations on all reactions. Obtain the mass of the unisolated first intermediate. Obtain a mass of the crude final product and determine overall percent yield. Obtain a % recovery for the recrystallization and a final melting point Comments: Yield: What is the theoretical yield of the first step of the reaction? What was your percent yield for the first step? Is it efficient? What is the overall yield of this three step reaction sequence? Intermediates: What color changes did you observe for the reaction? Use Molecular Orbital theory to explain how these color changes are consistent with the structures of the compounds. (See Brown and Foote Chapter 20) Mechanism: Provide a mechanism for each of the three steps of the reaction. Green chemistry: Give 5-6 characteristics of a “green” reaction and describe how well this synthesis fits a model of sustainability. Calculate the atom efficiency for this three step sequence. Calculate the Environmental factor (E-factor) as defined by the ratio of waste (in Kg) divided by product (in Kg) for this experiment. What factors did you consider in calculating the E-factor? Formal report: Cite the following two articles in your introduction, and incorporate them into your discussion. Pure and applied Chemistry Volume 84, number 3 starting on page 827. Use this article to discuss some of the metrics that can be used to measure how “green” a reaction is. Also refer to this article to give examples of how the study of green chemistry is used in an industrial example. Synthetic Communications Volume 42 number 19 starting on page 2831. Use this article as an example of how current research is being conducted to develop more eco-friendly catalysts. From the article, explain what they were trying to accomplish, and how well they accomplished their goal.