Reductive Amination & Acylation: Lab Synthesis
advertisement

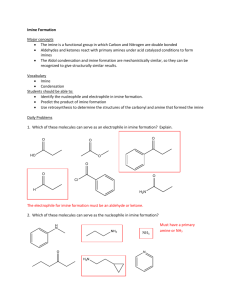
Reductive Amination and Acylation OH H MeO OH O + p-toluidine EtOH an imine O OH NaBH 4 N H 2N o-vanillin O OH O MeO H MeO MeO N H N O an amine an amide Background Reductive amination is a common strategy for the synthesis of amines. An aldehyde or ketone is condensed with an amine to form an imine, which is then reduced to the corresponding amine (usually by a borohydride reagent1): R2 R1 O + H 2N R1 R2 [H] R2 R3 N R3 R1 N H R3 Although this reaction can be performed in two steps (imine formation followed by borohydride reduction), it is common to perform the sequence in “one pot”. However, this introduces a potential complication: under the reaction conditions, imine formation is a reversible process, and many reducing agents such as sodium borohydride reduce aldehydes and ketones as well as imines. To avoid the direct reduction of your starting carbonyl compound to the corresponding alcohol, sodium cyanoborohydride (NaBH3CN) is commonly used because it chemoselectively reduces iminium ions over aldehydes or ketones. However, hydrogen cyanide is liberated when the reaction is quenched, which is highly toxic. Another chemoselective reducing agent that has been used is sodium triacetoxyborohydride (NaBH(OAc)3), but it is unstable in hydroxylic solvents such as methanol and water. 1 In the reaction you will be performing, the reductive amination will be performed in the same vessel (a beaker), but stepwise. Your first step, imine formation, is not performed in the typical manner. In this particular case, it turns out no solvent is required; the two solid reactants are blended together “neat”, which causes them to melt into a liquid. This is an extreme example of “melting point depression”, where each solid acts as an impurity to lower the melting point of the other solid; this is the same principle that the “mixed melting point” technique relies on. Solvent-free reactions, when possible, are of particular interest to industry. When solvents can account for up to 90% of the chemicals required for a process, a solvent-free varient could offer a substantially “greener” alternative.2 In the second step, the crude imine is dissolved in ethanol and treated with sodium borohydride. Because essentially only the imine remains after the reaction of your aldehyde and ketone, chemoselectivity is not an issue and borohydride may be used for this reduction. Although it is possible to isolate your amine product, you will be carrying this sequence one step further and converting your amine to the corresponding acetamide. A common way to synthesize esters and amides is to react an alcohol or amine with an “acylating agent”: an acyl group (RC=O) attached to a leaving group Y. Acid chlorides (Y = -Cl) and acid anhydrides (Y = -O2CR) are the most commonly used acylating agents. O R OH or R" O R" Y + HY O R NH R' R O R N R" R' 2 Your reduction reaction mixture, after the acetic acid quench, is treated with acetic anhydride and briefly heated. Whereas an esterification with an acid chloride or anhydride normally requires aprotic conditions (because the acylating agent reacts with solvents such as water or ethanol about as readily as it does with the reactant alcohol), amide formation is possible under protic conditions because the amine nitrogen is much more nucleophilic than a hydroxyl oxygen. For example, the classic Schotten-Baumann conditions are biphasic, where the amine and an acid chloride are dissolved in an organic phase and stirred with a basic aqueous phase. O NH + O NaOH(aq) Cl N 90% A typical Schotten-Baumann acylation3 3 Experimental Procedure SAFETY INFORMATION •Wear nitrile gloves and goggles throughout the entire experiment. Perform all chemical operations in the fume hoods. •p-toluidine is a suspected carcinogen, and toxic. Weigh in the fume hood. •Acetic acid and acetic anhydride are lachrymators. Use in the fume hood. •Sodium borohydride reacts with acids and protic solvents to form flammable hydrogen gas. Use in the fume hood. Destroy any excess reagent carefully with 1M HCl, then place in the aqueous waste carboy. •Ethanol and hexanes are flammable solvents. Synthesis of 2-methoxy-6-(p-tolyliminomethyl)-phenol: Imine formation Weigh a 250 mL beaker and then add 0.76 grams (5 mmol) of ortho-vanillin. Record the total mass of the beaker plus the ortho-vanillin. Using weighing paper, and in the fume hood, accurately weigh an equivalent amount of para-toluidine (0.535 grams, 5 mmol) and add this to the beaker. Observe this mixture for a few minutes and record what is happening. Using a heavy glass-stirring rod, mix and grind the solids until they become a homogeneous dry powder. Weigh the beaker and record the mass.4 Determine the percent yield. Synthesis of N-(2-hydroxy-3-methoxybenzyl)-p-methylaniline: Reduction of the Imine Add about 15 mL of 95% ethanol to the beaker containing your imine product; add a magnetic stir bar and stir the mixture to partially dissolve the imine. Weigh out approximately 0.1 g of sodium borohydride and slowly add this to the beaker in small increments with continued stirring. Record all observations and explain what is occurring in the reaction. Slowly add 2 mL of acetic acid to the reaction mixture to destroy the excess borohydride and to neutralize the phenoxide ion. 4 Synthesis of N-(2-hydroxy-3-methoxybenzyl)-N-p-tolylacetamide: Acetylation of the Amine Prepare a shallow hot-water bath. Add 2 mL of acetic anhydride to the beaker containing your reaction mixture, then warm the beaker in the hot-water bath while stirring for 5-10 minutes. Move this beaker to a cool stir plate, and stir the solution fairly rapidly while slowly adding 75 mL of water. As the alcohol and acetic acid are diluted the amide product should precipitate. Cool the mixture in an ice bath and collect the solid. Allow it to air-dry overnight and then obtain the mass of product. Analyze your product by IR spectroscopy and melting point (expected: 127128 °C). A small sample may be recrystallized from hexanes if desired. Your TA will select one student’s product for NMR analysis. 1 Other reducing agents may be used. In the TV series Breaking Bad, Walter White’s synthesis of methamphetamine supposedly used an aluminum amalgam and a mysterious set of reaction conditions that resulted in a highly enantioselective reduction. Jyllian Kemsley: “C&EN Talks With Donna Nelson”. Chem. Eng. News 2013, 91(38), 32. http://cen.acs.org/articles/91/i38/CENTalks-Donna-Nelson.html 2 Dicks, A.P. “Elimination of Solvents in the Organic Curriculum”. In Green Organic Chemistry in Lecture and Laboratory, CRC Press 2012. 3 Marvel, C.S.; Lazier, W.A. Organic Syntheses; Wiley & Sons: New York, 1941; I, 90. 4 For your conditions, imine formation occurs nearly quantitatively. However, the crude yield will be inflated because some of the water that is generated as a byproduct remains in the beaker. 5