CLINIC: - Acusis
advertisement

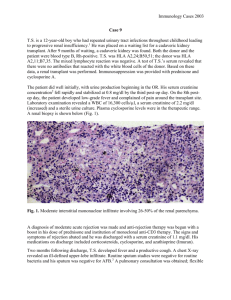
CLINIC: VISIT DATE: ATTENDING PHYSICIAN: Pediatric Renal Transplant Clinic 08/03/2006 Minnie M Sarwal, M.D., Ph.D. IDENTIFICATION: The patient is a 14-year-old male with endstage renal disease secondary to medullary cystic renal disease status post livingdonor renal transplant on 04/12/2006. He is attending today for a posttransplant scheduled clinic visit. Three-month protocol biopsy for the patient revealed evidence of Banff 1B rejection with B-cell infiltrates, and he was admitted and treated in-house with Solu-Medrol pulses and commenced on a course of rituximab therapy. He has received 2 doses and will receive his 3rd dose today. He is also on the steroid avoidance therapy and continues to receive an extended dose of Zenapax until the 6th posttransplant month. His creatinine remains stable at 0.9. Following his rejection episode, his CellCept axis has increased from 500 mg to 50-500 mg b.i.d. Donor-specific antibodies have been checked. The results of these are as of yet unavailable. REVIEW OF SYSTEMS: No specific complaints of fevers, nausea, vomiting, headache, diarrhea, or dysuria. No gastrointestinal intolerance with increased CellCept dosing. Remainder of review of systems is unremarkable. No allergies of note. FAMILY AND SOCIAL HISTORY: Unchanged. MEDICATIONS: 1. Prograf 6 mg in the morning, 5.5 mg in the evening. 2. CellCept 500 mg b.i.d. 3. Aspirin 81 mg daily. 4. Valcyte 450 mg daily. 5. Septra 1 single strength daily. 6. Sodium bicarbonate 650 mg t.i.d. LABORATORY DATA: Most recent labs from 07/27/2006: creatinine 0.9, BUN 12. Labs from today are pending. PHYSICAL EXAMINATION: VITAL SIGNS: Weight is 33.6 kg. Height is 140.2 cm. Blood pressure is 111/68. HEENT exam unremarkable. NECK: Supple, with no adenopathy or masses. CHEST: Clear to auscultation. Heart sounds normal, with no murmurs. ABDOMEN: Soft. Allograft firm, nontender, no overlying bruit. Midline scar. EXTREMITIES: Clear of edema. MUSCULOSKELETAL: Unremarkable. NEUROLOGIC: Grossly unremarkable. GU: Deferred. ASSESSMENT: The patient is status post surveillance biopsy-proven Banff 1B rejection with B-cell infiltrates. Stable creatinine running around 0.7 to 0.8 mg/dL, with the last creatinine more elevated at 0.9 mg/dL. Repeat labs from today are pending. He has mild metabolic acidosis. We made the following recommendations today: 1. Check his bicarbonate from today. If normalizing, consider discontinuing sodium bicarbonate. 2. Nurse visit next week and M.D. visit the week after. 3. Complete course of Rituxan to a total of 4 weekly doses. 4. Consider surveillance biopsy in early October to follow resolution of biopsy-proven rejection. 5. Check NPA level. If low, consider further increasing his CellCept axis, as the patient's baseline immunosuppression has always been stable and his rejection implies failure of his maintenance immunosuppression. 6. Continue to check donor-specific antibodies monthly, as well as CMV and EBV PCRs monthly.