Ferrofluid Activity
advertisement

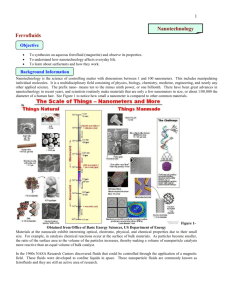
Materials Science Synthesis of an Aqueous Ferrofluid Ferrofluids are materials with the fluid properties of liquids and the magnetic properties of a solid. They contain tiny particles (~10 nm in diameter) of a magnetic solid suspended in a liquid medium. These particles must be small in order to remain suspended in the liquid medium. The most effective ferrofluids are made from particles of magnetite, Fe 3O4. Magnetite particles are made by combining Fe3+ and Fe2+ salts in a basic solution: 2 FeCl3 + FeCl2 + 8 NH3 + 4 H2O → Fe3O4 + 8 NH4Cl The slow addition of NH3 leads to the formation of nano-sized particles. However, these magnetite particles are attracted to each other by London dispersion forces. If left untreated, they will agglomerate into larger particles and precipitate from the liquid medium. In order to keep the magnetite particles separated, they are coated in a surfactant such as tetramethylammonium hydroxide (Fig. 1). Fig. 1 The hydroxide ions in this surfactant are attracted to the surface of each magnetite particle, forming a negatively charged layer at the magnetite surface. The tetramethylammonium cations are attracted to this negatively charged layer, forming a positive layer. When magnetite particles approach each other the repulsions between their positively-charged layers keeps them from getting too close (Fig. 2). N(CH3)4+ Fe3O4 Fe3O4 OHrepulsion between (+) charges keeps Fe3O4 particles separated Materials 1.0 M FeCl3 in 2 M HCl 2.0 M FeCl2 in 2 M HCl 1.0 M NH3 25% tetramethylammonium hydroxide in water deionized water in a wash bottle 100 or 150 mL beaker Fig. 2 stir plate and magnetic stirbar buret, buret clamp, and ring stand plastic weighing boat strong craft magnet glass stirring rod 10 mL graduated cylinder Reference: Adapted from: http://mrsec.wisc.edu/Edetc/nanolab/ffexp/index.html Procedure 1. Add 4.0 mL of 1.0 M FeCl3 and 1.0 mL of 2.0 M FeCl2 solution to a 100 or 150 mL beaker. Add a magnetic stirring bar and begin stirring on a stir plate. 2. While stirring, SLOWLY add 50 mL of 1.0 M aqueous NH3 solution by dripping it from a buret. This addition should last about 5 minutes. After an initial brown precipitate, a black precipitate will form (magnetite). 3. Remove the beaker from the stir plate. Immediately use a strong magnet on the OUTSIDE of the beaker to work the stir bar up the walls of the beaker. Remove the stir bar with tongs or a gloved hand BEFORE it touches the magnet. Rinse off the stir bar with water into a waste container, and clean it with a paper towel. 4. Let the magnetite in the beaker settle. You can speed the settling process by putting a magnet under the beaker. 5. Decant (pour off) and discard the clear liquid from the beaker without losing a substantial amount of solid. This works best if you keep a magnet under the beaker while you pour. 6. Transfer the solid to a plastic weighing boat with the aid of a few squirts of water from a wash bottle. 7. Put a strong magnet under the weighing boat to attract the ferrofluid to the bottom of the weighing boat. 8. Pour off and discard as much clear liquid as possible, again keeping the magnet under the weighing boat. Rinse with water from a wash bottle and decant the rinse as before. 9. Use a 10-mL graduated cylinder to add 1-2 mL of 25% tetramethylammonium hydroxide. Gently stir with a glass rod for at least a minute to suspend the solid in the liquid. Use a strong magnet to attract the ferrofluid to the bottom of the weighing boat. Pour off and discard the dark liquid. Move the strong magnet around and again pour off any liquid. If the ferrofluid does not spike, continue to move the strong magnet around, pouring off any liquid. 10. What happens when you move a magnet under the ferrofluid? If you are using a very strong magnet you might get more interesting results by varying the distance of the magnet below the boat. Cleanup: Rinse the weighing tray off with water (into a waste container) and dispose of it in the trash can. Pour all aqueous waste into the “metal cation waste” container. Conclusion Questions (answer in your lab notebook) 1. Were you able to prepare ferrofluid? Describe what you observed. 2. How many spikes were immediately around the central spike and how were they arranged? 3. Examine the starting FeCl2 and FeCl3 solids used to prepare magnetite (leave the solids in the sealed zip-loc bags.) How do they respond to a magnet? 4. What is the purpose of the surfactant? Explain chemically how the surfactant works to keep the nanoparticles suspended. 5. Why is it important that the magnetite particles be nano-sized?