Calculating Equilibrium Concentrations
advertisement
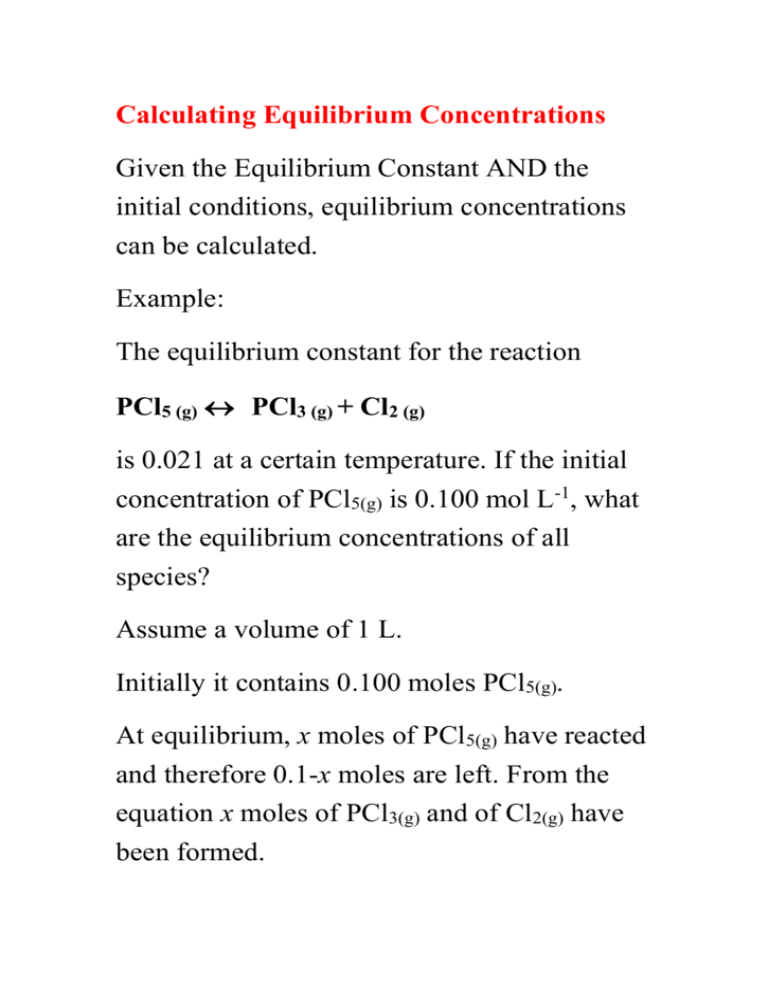
Calculating Equilibrium Concentrations
Given the Equilibrium Constant AND the
initial conditions, equilibrium concentrations
can be calculated.
Example:
The equilibrium constant for the reaction
PCl5 (g) PCl3 (g) + Cl2 (g)
is 0.021 at a certain temperature. If the initial
concentration of PCl5(g) is 0.100 mol L-1, what
are the equilibrium concentrations of all
species?
Assume a volume of 1 L.
Initially it contains 0.100 moles PCl5(g).
At equilibrium, x moles of PCl5(g) have reacted
and therefore 0.1-x moles are left. From the
equation x moles of PCl3(g) and of Cl2(g) have
been formed.
The Equilibrium Concentrations are:
[PCl5(g)] = (1-x) mol L-1, [PCl3(g)] = x mol L-1,
[Cl2(g)] = x mol L-1
K = [PCl3(g)] [Cl2(g)] / [PCl5(g)] = x . x /(0.1 – x)
= 0.021
Solve quadratic equation, x = 0.0365 mol
Therefore at Equilibrium: [PCl5(g)] = 0.063
mol L-1,
[PCl3(g)] = 0.037 mol L-1, [Cl2(g)] = 0.037 mol
L-1
Calculate the equilibrium concentrations when
0.50 mol L-1 HF(g) decomposes given:
2HF(g) H2 (g) + F2 (g)
K=
1.0x10-13
initial
mol L-1
0.50
0
0
equil.
mol L-1
0.5 – x
x
x
K = [H2 (g)] [F2 (g) ] / [ HF(g) ]2 = x2 / (0.5 – x)2
= 1.0x10-13
Clearly x << 0.5: Therefore: x2 / (0.5 )2 `
1.0x10-13
Solve to find: x = 1.6 x 10-7 mol L-1 (which is
<< 0.5)
Therefore: [H2 (g)] = [F2 (g)] = 1.6 x 10-7 mol L-1
and [HF (g)] = 0.50 mol L-1, at equil.
Have to be careful using the approximation
that x is very small. If used in the first example
the answer would have been in error by 30%
!!!
More examples:
2NO (g) + Cl2 (g)
2NOCl (g) , K = 4.6x104
Calculate the equilibr`ium concentrations of all species
when:
a) Initial concentration [NOCl (g) ] = 2.0 mol L-1
initial
equil.
2NO (g) + Cl2 (g)
0
0
2x
x
2NOCl (g)
2.0
mol L-1
2.0 - 2x mol L-1
K = [NOCl (g)]2 / [NO (g)]2 [Cl2 (g)] = ( 2.0 - 2x)2/(2x)2x
assume x << 2.0, K = 2.02/4x3 = 4.6x104
Solve: x = 0.028 mol L-1 (which is <<2.0)
[NOCl (g)] = 2.0 – 2x = 1.944 mol L-1
[NO (g)] = 2x = 0.056 mol L-1
[Cl2 (g)] = x = 0.028 mol L-1
(b) Initial Concentration [NO (g)] = 2.0mol L-1,
[NOCl (g)] = 2.0 mol L-
1
2NO (g) + Cl2 (g)
initial 2.0
equil. 2.0+2x
0
x
2NOCl (g)
2.0
2.0 - 2x
mol L-1
mol L-1
K = [NOCl (g)]2 / [NO (g)]2 [Cl2 (g)]
= ( 2.0 - 2x)2 / (2.0+x)2 x assume x << 2.0,
K = 4 / 4x = 4.6 x 104
x = 2.17 x10-5 mol L-1 (<< 2.0)
[NOCl (g)] = 2.0 - x = 2.0 mol L-1
[NO (g)] = 2.0 + x = 2.0 mol L-1
[Cl2 (g)] = x = 2.2 x 10-5 mol L-1
ACIDS AND BASES
Important application of equilibria in aqueous solutions.
Brønsted definitions:
ACID - a compound that donates a proton, H +
BASE - a compound that accepts a proton
Clearly Acids react with Bases!
e.g.
HNO3 (aq) + NH3 (aq)
Acid
Base
NO3-(aq) + NH4+ (aq)
In the reverse direction, NH4+ (aq) is the acid and NO3-(aq)- is the base.
Called the Conjugate Acid and the Conjugate Base.
e.g.
HCl (aq) + NaOH (aq)
NaCl (aq) + H2O (aq)
Ignore the Na+ (aq) ions:
HCl (aq) + OH- (aq)
Acid
Base
Cl- (aq)
Conj. Base
+ H2O (aq)
Conj. Acid
Water can act as a Base:
HNO3 (aq) + H2O (aq)
NO3-(aq) + H3O + (aq)
H3O + (aq) – the hydronium ion
HNO3 , Nitric Acid, is a strong acid. All reactions involving strong acids
on the LHS go completely to the RHS. K is very large.
Strong Acids: HCl, HBr, HI, HClO4, HNO3 and
H2SO4 for the donation of 1 proton:
H2SO4 (aq) + H2O (aq)
Other Acids are weak acids:
e.g.
HNO2 (aq) + H2O (aq)
HSO4-(aq) + H3O + (aq)
NO2-(aq) + H3O + (aq)
Reaction in equilibrium and HNO2 (aq) – nitrous acid – is only partially
dissociated, K is small.
For a general aqueous acid:
HA (aq) + H2O (aq)
A-(aq) + H3O + (aq)
K = [A-(aq) ][ H3O + (aq)] / [HA (aq) ][ H2O (aq)]
But, [ H2O (aq)] is a constant
Define, the Acid Constant:
Ka = [A-(aq) ][ H3O + (aq)] / [HA (aq) ]
There are many weak acids. Common ones are:
HF (aq) ,
HNO2 (aq) , HCN (aq) H3-PO-4 (aq)
-4
Ka 6.7x10
4.5x10-4
4x10-10 7.5x10-4 at
25oC
Organic Acids thousands of them, Ka < 10-5
Anions of polyprotic acids:
HSO4-(aq)
H-2PO4- (aq)
1.2x10-2
6.3x10-8 at 25oC
Calculate the percentage dissociation of 0.3 mol L-1 HF(aq) at
25oC
HF (aq) + H2O (aq)
Initial: 0.3
Equil: 0.3-x
F-(aq) + H3O + (aq)
0
x
0
x
mol L-1
mol L-1
Ka = [F-(aq) ][ H3O + (aq)] / [HF (aq) ] = x. x / (0.3-x)
= 6.7x10-4
assume x << 0.3, x2/0.3 = 6.7x10-4 , x = 1.4 x10-2 mol L-1
Therefore 1.4 x10-2 mol L-1 have dissociated:
Therefore % age dissociation = {(1.4x10-2)/0.3}x100
= 4.7 %
Note: x < 0.3 but not greatly so.
Approx gives x = 1.41 x10-2 , Exact gives x = 1.38 x10-2