Quantitative Analysis (CHM 235)
advertisement

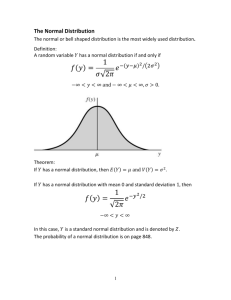
Quantitative Analysis (CHM 235) Dr. S.A. Skrabal Exam 1—26 Sept. 2006 Name:_____________________________________ Instructions: Read each question carefully. Show calculations when required to receive full credit. It is not necessary to show work for the mean and standard deviation when your calculator functions are used. Partial credit is given when warranted. Please box in your final answer. Points will be taken off for incorrect significant figures. Value of each question is given in parentheses after the question. Total points = 100. Note that important information is given on the back pages of the exam. Good luck! 1. Sally in the quant lab prepares a solution of aluminum trichloride (AlCl3) for use in an experiment. She pipets 2.50 (± 0.01) mL of 10.0 (± 0.2) µM AlCl3 into a volumetric flask and dilutes the solution with deionized water to a final volume of 100.0 (± 0.1) mL. (a) What is the concentration of AlCl3 in the final diluted solution in units of µM? (7) (a) Cconc Vconc = Cdil Vdil Cconc = (Cdil Vdil) / Vconc = (10.0 μM) (2.50 mL) / (100.0 mL) = 0.250 μM (b) What are the absolute and relative uncertainties of the concentration in the final diluted solution? (10) [10.0 (± 0.2) μM] [2.50 (± 0.01) mL] / [100.0 (± 0.1) mL] = 0.250 μM ± ? Convert all the absolute uncertainties to relative uncertainties: (0.2/10.0)100 = 2.0% (0.01/2.50)100 = 0.40% (0.1/100.0)100 = 0.10% %e (2.0 %) 2 (0.4 0 %) 2 (0.10 %) 2 2.0 % Convert back into absolute uncertainty and use “real rule” of sig. figs. (2.0%) (0.250 μM) = (0.020) (0.250 μM) = 0.005 μM Final answers: 0.250 ± 0.005 μM and 0.250 μM ± 2.0% (c) What is the concentration of Cl- in the final diluted solution in units of parts per billion? (5) Remember 1 ppb = 1 μg L-1 (0.250 μmol/L)(1 mol/106 μmol)(3 mol Cl-/1 mol Al)(35.45 g Cl-/mol Cl-)(106 μg/g) = 26.58 or 26.6 μg L-1 or ppb 2. What is the molality (m) of a 10.0 %(w:w) solution of sodium bromide (NaBr)? (10) Consider 100 g of this solution. 10.0 %(w:w) means 10.0 g NaBr/100 g soln. In 100 g of solution, therefore, there are 10.0 g NaBr and 90.0 g H2O, which is the solvent. Since molality = mol solute/kg solvent, we have (10.0 g NaBr/90.0 g H2O) (1000 g/kg) (1 mol NaBr/102.89 g NaBr) = 1.08 mol NaBr/kg H2O or 1.08 m 3. The recent doping controversy regarding Floyd Landis, the 2006 Tour de France winner, is centered on the unnaturally high ratio of to testosterone to epitestosterone found in his urine. High ratios, above 4:1, suggest that doping with the male hormone testosterone has occurred. (Landis’s sample showed a ratio of 11:1.) Suppose that during an interlaboratory comparison exercise, two testing laboratories, one in France and one in Belgium, have been given portions of the exact same urine sample to analyze for the ratio of the concentrations of testosterone to epitestosterone. In 5 analyses, the French lab obtains a mean ratio of 7.41 with a standard deviation of 0.75. In 7 analyses, the Belgian lab obtains a mean ratio of 8.52 with a standard deviation of 0.56. (a) Use the F-test to determine whether or not the standard deviations (i.e., the precisions) of the two sets of measurements are significantly different at the 95% confidence level. (5) Fcalc s12 s 22 (0.07 5 ) 2 (0.05 6 ) 2 1.79 df = (5 -1) = 4 for s1 and (7- 1) = 6 for s2. F(df = 4,6; 95% CL) = 4.53 Since Fcalc < Ftable, the standard deviations are not significantly different at the 95% CL. (b) Determine whether or not the testosterone-to-epitestosterone ratios obtained by the two laboratories are significantly different at the 95% confidence level. (10) s pooled t calc s12 (n1 1) s 22 (n2 1) n1 n2 2 x1 x 2 n1 n2 s pooled n1 n2 (0.7 5 ) 2 (5 1) (0.5 6 ) 2 (7 1) 572 7.41 8.5 2 0.6428 (7)(5) 75 0.6428 2.949 df = 5 + 7 – 2 = 10 t(df = 10, 95% CL) = 2.228 Since tcalc > ttable, the means are significantly different at the 95% CL. 2 4. The antidepressant Lexapro™ (escitalopram HBr) is manufactured in 10 mg tablets. Suppose that as part of a quality control program, 121 tablets of Lexapro 10-mg tablets were analyzed, yielding a mean drug content of 9.95 mg with a standard deviation of 0.094 mg. Calculate the 90% and 98% confidence interval for these analyses. (10) x ts n df = n -1 – 121 – 1 = 120 90% CI: ttable(df = 120, 90% CL) = 1.658 9.95 (1.658)(0.09 4 ) 121 9.95 0.014 or 9.95 0.014 mg 98% CI: ttable(df = 120, 98% CL) = 2.358 9.95 (2.358)(0.09 4 ) 121 9.95 0.020 or 9.95 0.02 0 mg 5. The National Research Council of Canada sells a standard reference material (SRM) for metals in dried sediment called MESS-3. Suppose you analyze the MESS-3 SRM for cadmium (Cd) content. For eight analyses, you obtain the following results (in mg kg-1): 0.21, 0.12, 0.22, 0.19, 0.24, 0.23, 0.18, 0.21. The certified value for Cd in the SRM is 0.24 mg kg-1. (a) Test any possible outliers for elimination using the Q-test at the 90% confidence level. (7) Arranged data: 0.12, 0.18, 0.19, 0.21, 0.21, 0.22, 0.23, 0.24 mg kg-1 The questionable data point is 0.12. Qcalc = gap/range = (0.18 – 0.12) / (0.24 – 0.12) = 0.50 Q(90% CL, n = 8) = 0.468 Since Qcalc > Qtable, data point can be rejected at the 90% CL. (b) Calculate the mean, standard deviation, and relative standard deviation of the acceptable data. (10) The rejected point is excluded from the following calculations. By calculator: Mean = 0.211 mg kg-1 Std. dev. = 0.021 mg kg-1 RSD = (0.021 / 0.211) 100 = 9.9 % or 1 x 101 % (c) Determine whether or not you are obtaining a Cd concentration in the SRM that is significantly different from the certified value at the 90% confidence level. (10) t calc known value x s n 0.24 0.211 0.021 7 3.654 df = n – 1 = 7 – 1 = 6 t(df = 6, 90% CL) = 1.943 Since tcalc > ttable, the obtained value is significantly different that the certified value at the 90% CL. 3 6. A student prepared a set of standards for dissolved aluminum ranging in concentrations of 0 to 16 µM. These were analyzed using a spectrophotometric technique. Using EXCEL, the student plotted his results, then used the LINEST function to obtain the parameters of the regression equation, including the slope (m), the standard deviation of the slope (sm), the y-intercept (b), the standard deviation of the y-intercept (sb), and the standard deviation of the y values (sy). These results are shown below. [Al] (µM) Abs. Corr. Abs. 0 1 5 10 16 0.001 0.048 0.244 0.484 0.791 0.000 0.047 0.243 0.483 0.790 m= sd of m = R^2 = 0.0492596 0.0003783 0.9998231 16959.6848341 0.4299771 -0.0026614 0.0033062 0.0050352 3.0000000 0.0000761 =b = sd of b = sd of y (a) Write the linear regression equation in the form y (± sy) = m (± sm) x + b (± sb) using the correct number of significant figures. (8) Applying real rule on sig. figs.: y (± 0.0050) = 0.04925 (± 0.00037) x - 0.0026 (± 0.0033) or y (± 0.005) = 0.0493 (± 0.0004) x + 0.003 (± 0.003) (b) Suppose an unknown sample was analyzed for Al in the same manner as the standards, giving an absorbance of 0.538. Determine the Al concentration (in μM) of the unknown sample. (8) Rearrange equation to solve for x = conc. and y = absorbance Blank- corrected absorbance = 0.538 – 0.001 = 0.537 x y b m x 0.537 (0.002 6 ) 0.0492 5 10.9 5 or 11.0 M 4 Useful information Standard deviation: s ( xi x ) 2 s 100 x %RSD = i Confidence interval: x n 1 ts where degrees of freedom = n - 1. n Comparison of means (when standard deviations are not significantly different): t calc x1 x 2 n1 n2 s pooled n1 n2 s pooled s12 (n1 1) s 22 (n2 1) n1 n2 2 where degrees of freedom = n1 + n2 - 2 Comparison of means (when standard deviations are significantly different): t calc x1 x 2 s12 / n1 s 22 / n2 DF Comparing measured result to known result: ( s 2 / n s 22 / n2 ) 2 2 1 21 ( s 22 / n2 ) 2 ( s1 / n1 ) n1 1 n2 1 t calc known value x s 2 n where degrees of freedom = n – 1. Fcalc s12 s 22 where s1 > s2 and degrees of freedom is n1 - 1 and n2 - 1 en e12 e22 e32 ... en21 %en %e12 %e22 %e32 ... %en21 y = mx + b Q = gap/range 5