Nitrogen Removal in a Single-Chambered Microbial Fuel
advertisement
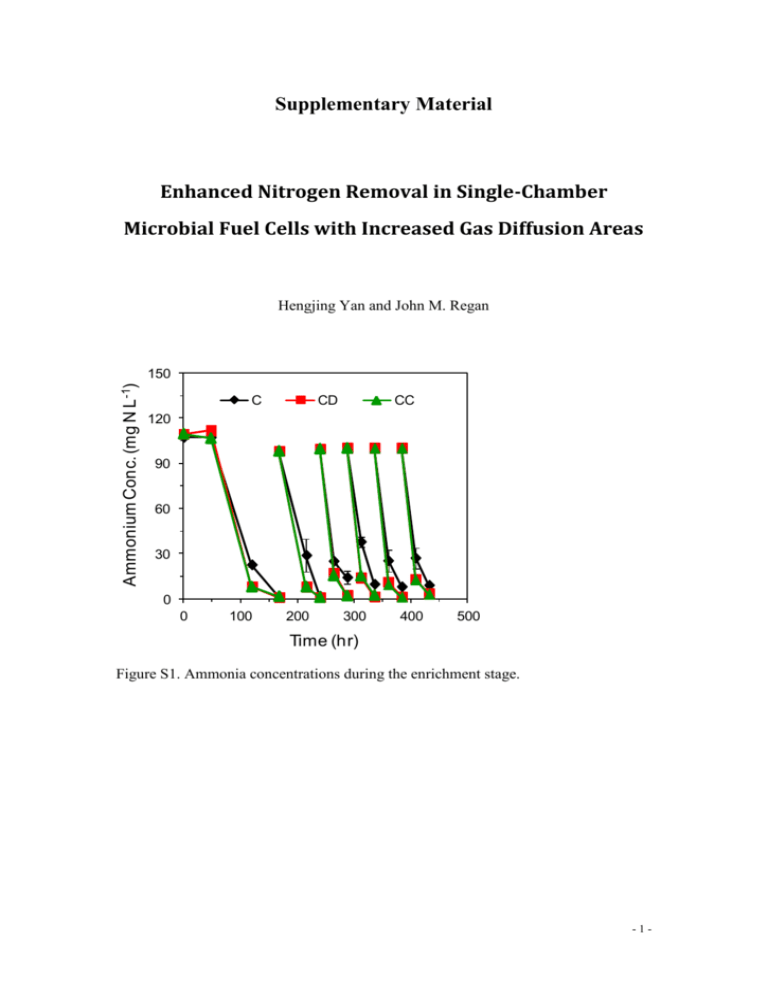
Supplementary Material Enhanced Nitrogen Removal in Single-Chamber Microbial Fuel Cells with Increased Gas Diffusion Areas Hengjing Yan and John M. Regan Ammonium Conc. (mg N L -1) 150 C CD CC 120 90 60 30 0 0 100 200 300 400 500 Time (hr) Figure S1. Ammonia concentrations during the enrichment stage. -1- Ammonia removal eff. (%) 100 80 60 40 20 C CD CC 0 0 2 4 6 8 10 12 14 16 Batch Figure S2. Ammonia removal efficiencies in C, CD, and CC reactors during fed-batch mode. 1.0 C Mean Voltage (V) 0.8 CD CC 0.6 0.4 0.2 0.0 1 3 5 7 9 11 13 15 COD: N Ratio Figure S3. Cell voltages (mean values at each COD/N ratio condition) of C, CD, and CC MFCs. -2- Estimation of ammonia assimilation rates using total redox reaction with cell synthesis for the combination of both non-exoelectrogens and exoelectrogens We estimated the ammonia assimilation rate for the COD/N ratio of 13, assuming that autotrophic assimilation was minor in comparison with heterotrophic assimilation and that methanogenesis was negligible. The stoichiometric relation between ammonia assimilation and COD removal rates is shown in (eq. S-1), where and respectively, are the molecular weights of nitrogen and sodium acetate, and are the stoichiometric coefficients for ammonium and sodium acetate in the total redox reaction with cell synthesis for the combination of both non-exoelectrogens growing on the air cathode and exoelectrogens, and 0.78 is the mass conversion factor from COD to sodium acetate. [eq. The S-1]. values for C, CD, and CC reactors are 0.216, 0.221, and 0.217, respectively. The detailed calculations are shown below. Electron balance: in our mixed-culture single-chamber MFC system, we assume that fraction a from the total acetate-derived electrons was used by exoelectrogens, with fs1 of this fraction used for cell synthesis and fe1 transferred to the anode for energy generation (Fig. S4). Therefore, is simply the Coulombic efficiency. Bacteria growing nonexoelectrogenically on the air cathode use the balance (i.e., 1-a) of the total acetate-derived electrons, with fs2 of this portion invested in cell synthesis and fe2 -3- transferred to oxygen. fs2 and fe2 were adopted from empirical values for aerobic heterotrophs (fs2 = 0.6 and fe2 = 0.4) (Rittmann and McCarty 2001). Figure S4. Chart of electron flow from the substrate acetate to different pathways in MFC systems. An energy balance between catabolism and anabolism was then coupled to the electron balance to calculate fs1, fe1, and a (Rittmann and McCarty 2001) . Energy balance: G p is the energy required to convert the sodium acetate to pyruvate, which is G p 35.09 27.40 7.69 kJ / e eq [eq. S-2]; G pc is the energy required to convert pyruvate to cellular carbon, estimated to be G pc 18.8 kJ / e eq [eq. S-3]; The term was used to account for energy–transfer efficiency. Therefore, the total energy requirement for cell synthesis ( Gs ) is Gs G p G pc [eq. S-4]. -4- Gr is the free energy released per each equivalent of sodium acetate oxidized for energy generation. To supply the energy required for cell synthesis ( Gs ), A equivalents of sodium acetate must be oxidized, which yields AGr . Assuming the same energy transfer efficiency as cell synthesis ( ), the energy balance is AGr Gs 0 [eq. S-5], A Gs Gr G p G pc Gr G p G pc 2 Gr 73.58 kJ / e eq [eq. S-6]. Gr At the anode, sodium acetate was oxidized to carbon dioxide and the electrons went from sodium acetate to the anode electrode. The free energy released from this process ( Gr ) could be calculated from the difference of the reduction potentials between the CO2/acetate redox couple ( ECO2 / acetate 0.28 V ) and the anode ( Eanode ) (Madigan 2006) as follows: Gr Gr RT ln 0' ([CO2 ])1/ 8 ([ HCO3 ])1/ 8 [ H ] ([ Ac ])1/ 8 ([CO2 ])1/ 8 ([ HCO3 ])1/ 8 [ H ] nFE0 RT ln ([ Ac ])1/ 8 ' ([CO2 ])1/ 8 ([ HCO3 ])1/ 8 [ H ] nF ( Eanode E ) RT ln ([ Ac ])1/ 8 1 (96485 C/mol ) ( Eanode (0.28 V)) ' 0 CO2 / acetate (8.314 J/(mol K)) (303.15K ) ln ([CO2 ])1/ 8 ([ HCO3 ])1/ 8 [ H ] . ([ Ac ])1/ 8 [eq. S-7] The values of Eanode , sodium acetate effluent concentration [ Ac ] , CO2 produced from the oxidation of the sodium acetate, [CO2 ] , [ HCO3 ] , and pH for C, CD, and CC reactors at a COD/N ratio of 13 are shown below: -5- C CD CC Eanode (V) -0.254 -0.244 -0.250 [ Ac ] (mol/L) 0.146 0.064 0.072 * [CO2 ] (mol/L) 0.0040 0.0053 0.0053 [ HCO3 ] (mol/L) 0.00048 0.00056 0.00054 pH 7.23 7.28 7.30 *The calculation of [CO2 ] and [ HCO3 ] : CO2 is produced from sodium acetate. We assume a portion X of the sodium acetate removed was converted to CO2 and HCO3-. Therefore, X a f e1 (1 a) f e2 CE f e2 a f e2 CE 0.4 0.4a [eq. S-8]. Since there is no headspace, we further assume that all CO2 was converted to H2CO3. Hence, we have at 30 °C: pKa [ HCO3 ][ H ] [ H 2CO3 ] 3 5.01187 10 7 (Snoeyink and Jenkins, 1980) [eq. S-9, 10] [ H 2CO3 ] [ HCO ] 2 X (Ac ) We used the trial and error method to solve this problem for a and the Using the relationships f e1 [CO2 ] and [ HCO3 ] values. A 1 , f s1 , and a CE / f e1 , the Gr , A , 1 A 1 A f e1 , f s1 , and a values of C, CD, and CC reactors were calculated as the following: C CD CC Gr (kJ/e- eq) -46.60 -47.72 -47.20 A 1.579 1.542 1.556 fe1 0.612 0.607 0.609 f s1 0.388 0.393 0.391 a 0.282 0.235 0.278 1 ' The vNH in the following cell synthesis reaction is : 4 20 -6- 1 1 1 1 9 CO2 HCO3- NH4 H e C5 H 7 O 2 N H O , and the 5 20 20 20 20 2 1 ' in the following reduction reaction is vCH : COO 3 8 1 1 1 3 CO2 HCO3- H e CH3COO- H 2O . 8 8 8 8 Therefore, the stoichiometric relationship between NH4 and CH3 COO- in the system is vNH 4 vCH COO 3 a ' f s1 vNH 4 ' vCH COO 3 (1 a) ' f s 2 vNH 4 ' vCH COO 3 , and the values were calculated as below: vNH vCH COO 4 3 C CD CC 0.216 0.221 0.217 -7-