Supplementary_Information
advertisement
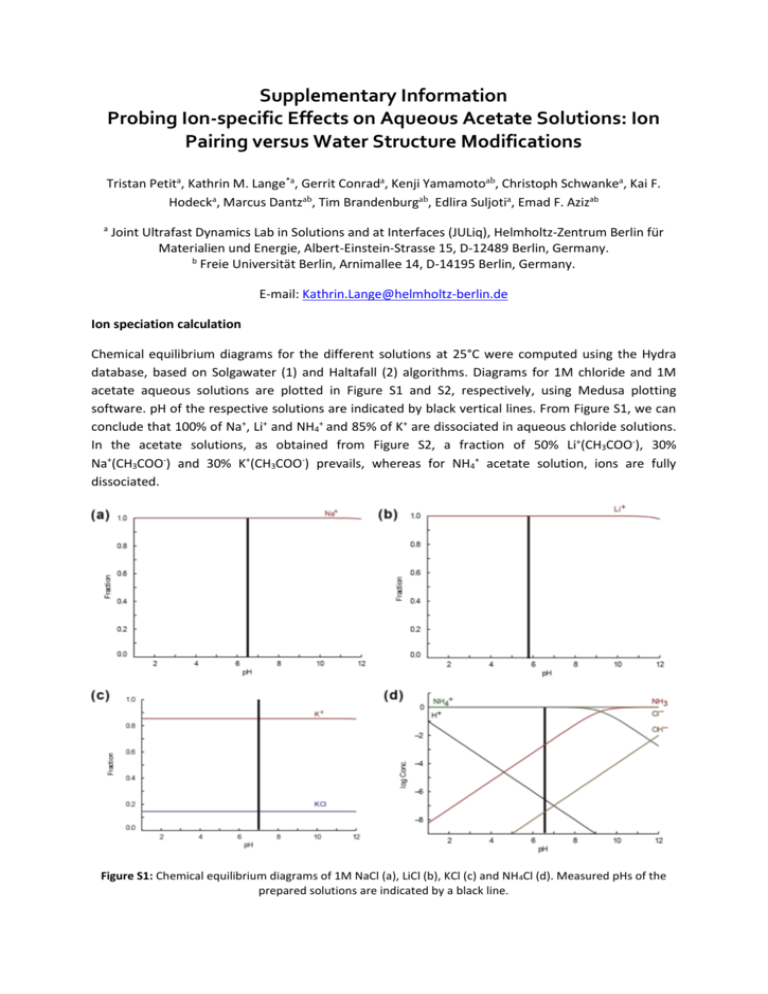
Supplementary Information Probing Ion-specific Effects on Aqueous Acetate Solutions: Ion Pairing versus Water Structure Modifications Tristan Petita, Kathrin M. Lange*a, Gerrit Conrada, Kenji Yamamotoab, Christoph Schwankea, Kai F. Hodecka, Marcus Dantzab, Tim Brandenburgab, Edlira Suljotia, Emad F. Azizab a Joint Ultrafast Dynamics Lab in Solutions and at Interfaces (JULiq), Helmholtz-Zentrum Berlin für Materialien und Energie, Albert-Einstein-Strasse 15, D-12489 Berlin, Germany. b Freie Universität Berlin, Arnimallee 14, D-14195 Berlin, Germany. E-mail: Kathrin.Lange@helmholtz-berlin.de Ion speciation calculation Chemical equilibrium diagrams for the different solutions at 25°C were computed using the Hydra database, based on Solgawater (1) and Haltafall (2) algorithms. Diagrams for 1M chloride and 1M acetate aqueous solutions are plotted in Figure S1 and S2, respectively, using Medusa plotting software. pH of the respective solutions are indicated by black vertical lines. From Figure S1, we can conclude that 100% of Na+, Li+ and NH4+ and 85% of K+ are dissociated in aqueous chloride solutions. In the acetate solutions, as obtained from Figure S2, a fraction of 50% Li+(CH3COO-), 30% Na+(CH3COO-) and 30% K+(CH3COO-) prevails, whereas for NH4+ acetate solution, ions are fully dissociated. Figure S1: Chemical equilibrium diagrams of 1M NaCl (a), LiCl (b), KCl (c) and NH4Cl (d). Measured pHs of the prepared solutions are indicated by a black line. Figure S2: Chemical equilibrium diagrams of 1M Na+ acetate (a), Li+ acetate (b), K+ acetate (c) and NH4+ acetate (d). Measured pHs of the prepared solutions are indicated by a black line. References (1) Eriksson G, 1979. An algorithm for the computation of aqueous multicomponent, multiphase equilibria. Anal. Chim. Acta, 112: 375-383. (2) Ingri N, Kakolowicz W, Sillén L G, Warnqvist B, 1967. High-speed computers as a supplement to graphical methods - V. HALTAFALL, a general program for calculating the composition of equilibrium mixtures. Talanta, 14: 1261-1286. Errata: 15(3) (1968) xi-xii.