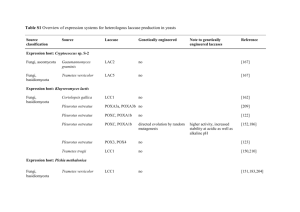
Table 1 Substrate specificity for purified laccase activity

Supplementary Information
Supplementary Methods
Fungal strain and culture conditions
Stock cultures of Pleurotus sp. (strain ‘MAK-II’) were maintained on the potato dextrose agar
(PDA) slant at 4 °C in the dark. The mycelium from the slant was transferred to PDA plates and incubated at 30 °C for 7 days. Mycelial discs from the peripheral region of actively growing culture were used as inoculum. The production medium contains (g l
-1
): 10.0 mannitol, 2.0 yeast extract, 2.0 L-proline, 0.3 erythromycin, 1.0 KH
2
PO
4
, 0.5 MgSO
4
, 0.01 CaCl
2
, 0.001 FeSO
4
, 0.1
Na
2
HPO
4
, 0.62 CuSO
4
, 0.0275 adenine, and 2.0 biotin in 1 l distilled water (pH 5.0). Laccase production was carried out under submerged fermentation in a laboratory scale bioreactor (5 l working volume capacity). Three liters of the production medium were added to the reactor and sterilized for 20 min at 121 °C at 15 psi. The pre-cultures were prepared in 250-ml Erlenmeyer flasks containing nutrient medium with glucose (20 g l
−1 ) in static conditions at 30 °C. Sevendays-old pre-cultures were homogenized before transferring into the medium in the bioreactor
(20 g l
−1
wet mycelium). The agitator and flow rate of filter-sterilized air were set at 100 rpm and
3 l h
−1
, respectively. The internal temperature of the bioreactor was maintained at 30 °C. After 3 days incubation, vanillic acid (1 mM) was added to the production medium as inducer to enhance laccase production.
Laccase gene amplification using degenerative primers from Pleurotus sp. MAK-II
Laccase gene amplification was performed with primers Cu1F: 5′-CAT(C) TGG CAT(C) GGN
TTT(C)TTT(C) CA-3′ and Cu2R: 5′-G G(A)CT GTG GTA CCAGAA NGT NCC-3′ according
to the method of D'Souza et al. (1996).
1
Effect of metal ions and various reagents on laccase activity
Different metal ions (1 mM) Al 3+ , Ca 2+ , Cd 2+ , Co 2+ , Cr 2+ , Cu 2+ , Fe 2+ , Mg 2+ , Mn 2+ , Na + , Ni 2+ ,
Hg
2+
, and Zn
2+ , or various inhibitors (1 mM; that included cyanide, β-mercaptoethanol, dithiothreitol, ethylenediaminetetraacetic acid, and sodium azide, or various surfactants (1% w/v and v/v; viz.: cetyltrimethylammonium bromide, Sarkosyl, SDS, Tween 20, Tween 80, and
Triton-X 100), or various solvents (5% v/v; that included acetone, acetonitrile, butanol, dimethyl sulfoxide, dimethylformamide, dichloromethane, ethanol, methanol, and isopropanol) were examined on laccase activity by pre-incubating the enzyme in above chemicals (metal ions, inhibitors, surfactants, and solvents ) for 1 h and the remaining enzyme activity was assayed under standard assay condition using 1 mM ABTS as substrate in sodium acetate buffer (100 mM, pH 4.5) at 30 °C. The activity assayed in the absence of any additives (metal ions or inhibitors or surfactants or solvents) was defined as 100%.
2
Supplementary Tables
Supplementary Table 1 Summary of purification steps of extracellular laccase from Pleurotus sp. MAK-II
Yield Purification step Total protein Total activity
(mg)
Culture supernatant 368.4
(Units)
82,000
104 44,800
Specific activity
(Units/mg protein)
222.5
430.7
Purification
(fold)
1
1.93
100
54.6 (NH
4
)
2
SO
4
Fraction
70% (w/v)
Sephadex G-100
DEAE cellulose
36
15
38,000
24,200
1055.5
1613.3
4.7
7.25
46.3
29.5
3
Supplementary Table 2 Comparison of physicochemical properties of laccase from Pleurotus sp. MAK-II and laccases from other white-rot fungi
Name of fungi Molecular mass (kDa)
Cerrena maxima 57.0 pI
3.5
Optimum pH Optimum
4.0-4.2 temperature
(°C)
50
Reference
–
3.0 55
Ganoderma fornicatum
Ganoderma lucidum
Ganoderma lucidum
Ganoderma lucidum
Ganoderma lucidum
Ganoderma lucidum
63.7
38.3
32.18
42.33
43.01
56.04
Ganoderma lucidum
57.17
Lentinula edodes 58.5
Pleurotus eryngii 60.0
Pleurotus eryngii 34.0
4.56
4.79
4.56
4.56
4.71
4.53
–
–
–
5
–
–
–
–
–
3.5
7
3-5
55
–
–
–
–
–
40
50
70
40.0 – 4 50
Pleurotus ostreatus
Pleurotus sajorcaju
Pleurotus sajorcaju
Pleurotus sajorcaju
Pleurotus sajor-
35.0
45.0
70.0
61.0
–
–
–
–
–
–
–
5.0
50
50
50
40
4
caju
Pleurotus sajorcaju
Pleurotus sp.
MAK-II
Pycnoporus sp.
SYBC-L1
Pycnoporus sp.
SYBC-L1
61.0
43.0
55.89
63.07
Trametes pubescens
Trametes pubescens
60.0
120.0
Trametes sp. Ha1 62.0
Trametes sp. Ha1 62.0
–
–
–
–
3.2
–
3.0
5.9
5
6.0
6.0
5.0
5.0
6.0
4.5
3
2.5
70
70
60
60
65
70
25
60
Present study
Supplementary Table 3 Substrate specificity for purified laccase activity
Substrate
ABTS
Guaiacol
Pyrogallol p -Phenylenediamine
Catechol
Absorbance at nm Relative activity (%)
436 100
436 98
450
487
450
82
55
38
Ferulic acid
Tyrosine
Veratryl alcohol
287
280
310
18
0
0
Values represent the mean of three independent experiments, with a maximal mean deviation of ± 5 %. Enzyme activity was measured under standard assay conditions, using ABTS as substrate.
6
Supplementary Table 4 Effect of different metal ions, inhibitors, surfactants, and solvents on purified laccase activity
Chemicals (concentration)
Control (None)
Mg 2+
Mn 2+
Na +
Ni 2+
Hg 2+
Zn 2+
Metal ions (1 mM)
Al 3+
Ca 2+
Cd 2+
Co 2+
Cr 2+
Cu 2+
Fe 2+
Inhibitors (1 mM)
Sodium azide
Cyanide
EDTA
DTT
β-Mercaptoethanol
Surfactants (1%, w/v and v/v)
SDS
Sarkosyl
CTAB
Tween 20
Tween 80
Triton-X 100
Solvents (5%, v/v)
Acetone
Acetonitrile
Butanol
Relative activity (%)
100
96
110
90
79
39
6
47
94
106
75
83
35
125
82
89
96
98
95
90
32
11
54
0
0
100
27
35
7
DMSO
DMF
DCM
Ethanol
92
51
67
98
Methanol
Isopropanol
95
97
Values represent the mean of three independent experiments, with a maximal mean deviation of ± 5 %. Enzyme activity was measured under standard assay conditions, using ABTS as substrate.
8
Supplementary Fig. 1 Chemical structures of the different dyes (a) Congo Red (diazo dye), (b)
Remazol Brilliant Blue R (anthraquinone dye) and redox mediators, (c) 1-hydroxybenzotriazole,
(d) p-coumaric acid, (e) violuric acid, and (f) syringaldehyde used in this study.
9
References
Bettin F, da Rosa LO, Montanari Q, Calloni R, Gaio TA, Malvessi E, da Silveira MM, Dillon
AJP (2011) Growth kinetics, production, and characterization of extracellular laccases from Pleurotus sajor-caju PS-2001. Process Biochem 46:758-764
D'Souza TM, Boominathan K, Reddy CA (1996) Isolation of laccase gene-specific sequences from white rot and brown rot fungi by PCR. Appl Environ Microbiol 62(10):3739-3744
Gaitan IJ, Medina SC, Gonzalez JC, Rodriguez A, Espejo AJ, Osma JF, Sarria V, Almeciga-Diaz
CJ, Sanchez OF (2011) Evaluation of toxicity and degradation of a chlorophenol mixture by the laccase produced by Trametes pubescens . Bioresour Technol 102:3632-3635
Huang WT, Tai R, Hseu RS, Huang CT (2011) Overexpression and characterization of a thermostable, pH-stable and organic solvent-tolerant Ganoderma fornicatum laccase in
Pichia pastoris . Process Biochem 46:1469-1474
Koroleva OV, Gavrilova VP, Stepanova EV, Lebedeva VI, Sverdlova NI, Landesman EO,
Yavmetdinov IS, Yaropolov AI (2002) Production of lignin modifying enzymes by cocultivated White-rot fungi Cerrena maxima and Coriolus hirsutus and characterization of laccase from Cerrena maxima. Enzyme Microb Technol 30:573-580
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685
Liu L, Lin Z, Zheng T, Lin L, Zheng C, Lin Z, Wang S, Wang Z (2009) Fermentation optimization and characterization of the laccase from Pleurotus ostreatus strain 10969.
Enzyme Microb Technol 44:426-433
10
Manavalan T, Manavalan A, Thangavelu KP, Heese K (2012) Secretome analysis of
Ganoderma lucidum cultivated in sugarcane bagasse. J Proteomics 77:298-309
Manavalan T, Manavalan A, Thangavelu KP, Heese K (2013) Characterization of optimized production, purification and application of laccase from Ganoderma lucidum . Biochem
Eng J 70:106-114
Murugesan K, Arulmani M, Nam IH, Kim YM, Chang YS, Kalaichelvan PT (2006) Purification and characterization of laccase produced by a white rot fungus Pleurotus sajor-caju under submerged culture condition and its potential in decolorization of azo dyes. Appl
Microbiol Biotechnol 72:939-946
Nagai M, Sakamoto Y, Nakade K, Sato T (2009) Purification of a novel extracellular laccase from solid-state culture of the edible mushroom Lentinula edodes . Mycoscience 50:308-
312 Nakatani M, Hibi M, Minoda M, Ogawa J, Yokozeki K, Shimizu S (2010) Two laccase isoenzymes and a peroxidase of a commercial laccase-producing basidiomycete,
Trametes sp. Ha1. New Biotechnol 27:317-323
Ueda M, Shintani K, Nakanishi-Anjyuin A, Nakazawa M, Kusuda M, Nakatani F, Kawaguchi T,
Tsujiyama S, Kawanishi M, Yagi T, Miyatake K (2012) A protein from Pleurotus eryngii var. tuoliensis C.J. Mou with strong removal activity against the natural steroid hormone, estriol: purification, characterization, and identification as a laccase. Enzyme Microb
Technol 51:402-407
Wang HX, Ng TB (2006) Purification of a laccase from fruiting bodies of the mushroom
Pleurotus eryngii . Appl Microbiol Biotechnol 69:521-525
11
Wang Z-X, Cai Y-J, Liao X-R, Tao G-J, Li Y-Y, Zhang F, Zhang D-B (2010) Purification and characterization of two thermostable laccases with high cold adapted characteristics from
Pycnoporus sp. SYBC-L1. Process Biochem 45:1720-1729
Zucca P, Rescigno A, Olianas A, Maccioni S, Sollai FA, Sanjust E (2011) Induction, purification, and characterization of a laccase isozyme from Pleurotus sajor-caju and the potential in decolorization of textile dyes. J Mol Catal B: Enzym 68:216-
222 http://dx.doi.org/10.1016/j.molcatb.2010.11.008
12