New 21st Century Chemistry
Suggested answers to in-text activities and unit-end exercises
Topic 12 Unit 41
In-text activities
Internet Search & Presentation (page 3)
Moseley’s periodic table
The modern periodic table lists the elements in order of increasing atomic number, i.e. the number
of protons in an atom. However, Mendeléev, who made the first periodic table in 1869, listed the
then-known elements in order of their relative atomic masses. This was because, at that time, the
idea of atoms being made up of smaller sub-atomic particles had not been developed.
By 1907, when Mendeléev died, chemists were in little doubt that iodine followed tellurium and
that their relative atomic masses were unusual.
In 1913, Moseley determined the wavelength and frequency of X-rays emitted by a large number
of solid elements when they were bombarded by a beam of electrons. By this time, the number of
protons in the nucleus of some of the lighter elements had been determined.
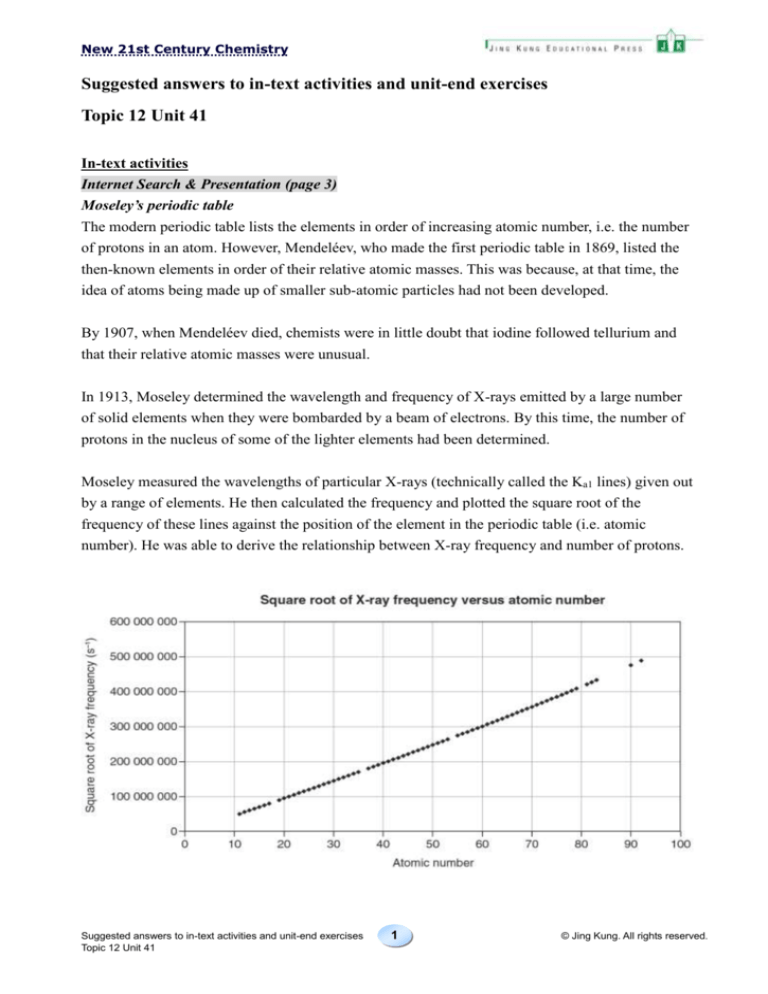
Moseley measured the wavelengths of particular X-rays (technically called the Ka1 lines) given out
by a range of elements. He then calculated the frequency and plotted the square root of the
frequency of these lines against the position of the element in the periodic table (i.e. atomic
number). He was able to derive the relationship between X-ray frequency and number of protons.
Suggested answers to in-text activities and unit-end exercises
Topic 12 Unit 41
1
© Jing Kung. All rights reserved.
New 21st Century Chemistry
Moseley’s results led to the conclusion that the order of elements in the periodic table was based
on some fundamental principle of atomic structure. As a result of Moseley’s work, chemists were
convinced that the periodic nature of the properties of elements is due to differences in the
numbers of subatomic particles.
Mendeléev’s periodic table was important because it enabled the properties of elements to be
predicted by means of the ‘Periodic Law’: properties of the elements vary periodically with their
atomic masses. The work of Moseley modified the 'Periodic Law' to read that the properties of the
elements vary periodically with their atomic numbers.
Moseley’s modified ‘Periodic Law’ puts the elements tellurium and iodine in the right order.
Seaborg — making new elements with sub-atomic particles
Seaborg with his co-workers identified 11 elements. These all have atomic number greater than 92
(uranium) and are man-made. These new elements led to an extension of the periodic table beyond
uranium.
The elements found by chemists before Seaborg were discovered — they existed on Earth already.
The elements found by Seaborg were actually made — they are elements that do not exist
naturally on Earth.
Scientists found that when they fired neutrons at uranium atoms, one would occasionally stick to a
uranium nucleus. Sometimes this neutron ‘spat out’ an electron and turned into a proton. This
meant that the nucleus now had 93 protons and was a new element, of atomic number 93, which
was named neptunium, Np. This was actually done by two other scientists. Seaborg then took over.
Bombardment with other sub-atomic particles allowed them to make elements numbers 94 – 103
in the same sort of way.
References:
http://www.rsc.org/Education/Teachers/Resources/periodictable/post16/order.doc
http://www.ausetute.com.au/pthistor.html
http://www.enotes.com/earth-science/periodic-table-predicting-structure-properties
http://www.rsc.org/Education/Teachers/Resources/periodictable/post16/discover.doc
Checkpoint (page 19)
1 a) Elements in the same group occur at similar positions on the graph, e.g. Group IV elements
(carbon and silicon) at peaks, Group 0 elements (helium, neon and argon) at troughs.
Suggested answers to in-text activities and unit-end exercises
Topic 12 Unit 41
2
© Jing Kung. All rights reserved.
New 21st Century Chemistry
b) To melt a metal, only a fraction of metallic bonds needs to be broken. All the remaining
metallic bonds are broken on boiling. Thus, the difference between the melting point and
boiling point of a metal is relatively larger.
To melt a non-metal, almost all the intermolecular forces need to be broken. Only a small
amount of heat is required to break the remaining intermolecular forces in the melted
non-metal upon boiling. Thus, the difference between the melting point and boiling point
of a non-metal is relatively smaller.
2
Sodium, magnesium and aluminium are all metals. They have metallic bonding.
Going from sodium to aluminium, the number of delocalized electrons increases. There are
more mobile electrons. So, the electrical conductivity increases.
In silicon, the four outermost shell electrons in each silicon atom are held in the four covalent
bonds which link the atom to four other silicon atoms.
Very few electrons gain enough energy to become delocalized. So, there are few delocalized
electrons and silicon is a poor electrical conductor.
The other elements in Period 3 from phosphorus to argon do not conduct electricity at all. This
is because they do not contain mobile electrons.
3
a) Element t has the highest boiling point and
there is a sudden drop in boiling point from t to u.
Thus, t should be carbon and u should be nitrogen.
b) Element y should be sodium while z should be magnesium. Both of them have metallic
bonding.
Suggested answers to in-text activities and unit-end exercises
Topic 12 Unit 41
3
© Jing Kung. All rights reserved.
New 21st Century Chemistry
Two electrons from each magnesium atom participate in metallic bond formation while
there is only one electron from each sodium atom.
The atomic size of magnesium is smaller than that of sodium and magnesium forms ions
carrying two positive charges. Thus, a magnesium ion has a larger charge density than a
sodium ion.
The electrostatic attractions between positive ions and electrons, and thus the metallic
bonds are stronger for magnesium than for sodium.
Discussion (page 21)
1 From the graph, we can see that density varies periodically:
• the alkali metals occupy the minimum positions at the beginning of each period;
•
•
the halogens or noble gases occupy the minimum positions at the end of each period;
the maxima are occupied by elements near the middle of each period: carbon, aluminium
and transition metals such as nickel and copper.
2
The density of an element is its mass per unit volume. It depends on its relative atomic mass,
atomic radius and degree of compactness.
The atomic radius of the three elements decreases in the order Na > Mg > Al.
The relative atomic mass of the three elements increases in the order Na < Mg < Al.
Thus, the density of the three elements increases in the order Na < Mg < Al.
3
The relative atomic mass of lead is much higher than that of germanium and
atoms in lead are more closely packed.
Thus, the density of lead is much higher than that of germanium.
Checkpoint (page 26)
a) NaCl
MgCl2
AlCl3
SiCl4
PCl5
SCl4 (formulae of other chlorides of sulphur are S2Cl2, SCl2 and S3Cl2)
b) Going across periods 2 and 3, there is a regular increase in the number of moles of chlorine
atoms that combines with one mole of atoms of each element. This number reaches a
maximum at Group IV or Group V.
c) Going down groups I to IV, the number of moles of chlorine atoms that combines with one
mole of atoms of elements in each group remains the same.
Suggested answers to in-text activities and unit-end exercises
Topic 12 Unit 41
4
© Jing Kung. All rights reserved.
New 21st Century Chemistry
Everyday Chemistry (page 27)
1 When heat is taken in, red phosphorus changes into white phosphorus which burns
spontaneously in air.
Thus, white phosphorus should be more reactive than red phosphorus.
2
Striking the match anywhere generates enough heat to ignite the phosphorus sulphide.
This initiates the decomposition of an oxidizing agent (such as potassium chlorate), liberating
the oxygen required for combustion.
The paraffin burns and carries the flame from the head to the wooden stem.
Checkpoint (page 31)
1 a) BaO(s) dissolves in the acid with heat released. A colourless solution is formed.
b) The solution turns milky, then clear again.
2
K2O is a basic oxide.
Ga2O3 is an amphoteric oxide.
Br2O5 is an acidic oxide.
3
a)
Oxide
Sodium oxide
Aluminium oxide
Sulphur trioxide
Formula of oxide
Na2O
Al2O3
SO3
Melting point (°C)
1 275
2 072
17
b) Both Na2O and Al2O3 have giant ionic structures with strong ionic bonds. A lot of heat is
needed to overcome the strong ionic bonds. Thus, they have high melting points.
Compared with Na+ ion, Al3+ ion has a higher charge and smaller size.
Thus, the ionic bond between Al3+ and O2– ions is stronger than that between Na+ and O2–
ions.
So, the melting point of Al2O3 is higher than that of Na2O.
SO3 has a simple molecular structure. Very little heat is needed to overcome the weak van
der Waals’ forces between the molecules. Thus, it has a much lower melting point.
c) Na2O dissolves readily in water to form a strongly alkaline solution.
Na2O(s) + H2O(l)
2NaOH(aq)
Al2O3 is insoluble in water.
SO3 dissolves readily in water to form a strongly acidic solution.
SO3(g) + H2O(l)
H2SO4(aq)
Suggested answers to in-text activities and unit-end exercises
Topic 12 Unit 41
5
© Jing Kung. All rights reserved.
New 21st Century Chemistry
Unit-end exercises (pages 34 – 42)
Answers for the HKCEE (Paper 1) and HKALE questions are not provided.
1
A possible concept map:
2
Element in
Na
Mg
Al
Si
P
S
Cl
Ar
Period 3
Type of
element
(metal /
metal
metalloid
non-metal
metalloid /
non-metal)
Type of
giant
giant metallic
simple molecular
structure
covalent
Nature of
metallic
covalent
bonding
dissolves
reacts
Reaction
in water
reacts
no
only with
with water
no reaction
to form
vigorously
reaction
steam
an acidic
solution
Suggested answers to in-text activities and unit-end exercises
Topic 12 Unit 41
6
© Jing Kung. All rights reserved.
New 21st Century Chemistry
3
a) Elements in the same group occur at similar positions on the graph, e.g. Group IV elements
(carbon and silicon) at peaks, Group 0 elements (helium, neon and argon) at troughs.
b) i) The same pattern
ii) All the values are higher.
c) Atomic radius or other correct suggestions
4
a) i) Cl
ii) Si
iii) Al
iv) Ar
b) Giant structure of SiO2
SO2
5
a) MgO(s) + H2O(l)
Mg(OH)2(s)
Magnesium oxide reacts slightly with cold water to form magnesium hydroxide which is
very slightly soluble.
So, a slightly alkaline solution is formed.
Suggested answers to in-text activities and unit-end exercises
Topic 12 Unit 41
7
© Jing Kung. All rights reserved.
New 21st Century Chemistry
SO2(g) + H2O(l)
H2SO3(aq)
Sulphur dioxide reacts with water to form sulphurous acid.
b) Silicon dioxide has a giant covalent structure.
The atoms are held together by strong covalent bonds and it is difficult to separate the
atoms.
6
C Option D — w represents phosphorus, y represents oxygen and z represents chlorine.
All of them exist as molecules under room conditions.
7
B Option B — Na, Mg and Al are metals. Their melting points are relatively high due to
their metallic bonding.
The strength of metallic bond increases from Na to Al due to the increasing
number of delocalized electrons for bonding and cationic charge density.
Silicon has a giant covalent structure.
A lot of heat is needed to overcome the strong covalent bonds between the
atoms. Hence it has a very high melting point.
Molecules in phosphorus, sulphur, chlorine and argon are attracted to one
another by weak van der Waals’ forces. Only very little heat is needed to
separate the molecules. Thus, they have low melting points.
The strength of van der Waals’ forces is in the order S8 > P4 > Cl2 > Ar. So,
the heat required to break the forces decreases in the same order, as shown
by their decreasing melting points.
8
D Option D —
chlorine is a non-metal. It forms oxides with molecular structures.
9
C Option C —
Oxides of metals are basic while those of non-metals are acidic.
Thus, Y should be a metal while X should be a non-metal. The order of
increasing atomic number of the three elements is Y < Z < X.
Suggested answers to in-text activities and unit-end exercises
Topic 12 Unit 41
8
© Jing Kung. All rights reserved.
New 21st Century Chemistry
10 B (1) Na, Mg and Al are all metals. They have metallic bonding.
Going from Na to Al, the number of delocalized electrons increases. There are more
mobile electrons.
So, the electrical conductivity increases from Na to Al.
(2) Moving from Na to Cl, electrons are added to the outermost shell. The charge on the
nucleus increases. The effective nuclear charge increases as we move across the period.
Electrons are pulled closer to the nucleus. So, the size of the atom decreases.
Thus, Cl has the smallest atomic radius.
(3) Molecules of P, S and Cl are attracted to one another by van der Waals’ forces.
The strength of van der Waals’ forces is in the order S8 > P4 > Cl2. The heat required to
break the forces and thus the melting point decreases in the same order.
11 a)
The boiling point of an element depends on the strength of attractions between its particles.
For Li, Na and K, one outermost shell electron of each atom is delocalized and available
for metallic bonding.
The atomic radii of the elements increase in the order Li < Na < K.
The attraction between the nucleus and delocalized electron decreases in the order Li > Na
> K.
Hence the strength of metallic bonding and the boiling point decrease in the order Li > Na
> K.
Suggested answers to in-text activities and unit-end exercises
Topic 12 Unit 41
9
© Jing Kung. All rights reserved.
New 21st Century Chemistry
b)
The melting point of an element depends on the strength of attractions between its
particles.
The number of delocalized electrons for metallic bonding increases in the order Na < Mg <
Al.
Their atomic radii decrease in the order Na > Mg > Al. Both Mg and Al have multi-charged
ions. Thus, their ions have larger charge density and the electrostatic attractions with
electrons are stronger.
The strength of metallic bonds and thus the melting points of the elements increase in the
order Na < Mg < Al.
12 a)
Element
Structure
Bonding
Mg
giant
metallic
Si
giant
covalent
S
simple
covalent
b) The boiling point of an element depends on the strength of attractions between its particles.
Silicon has a giant covalent structure. A lot of heat is needed to overcome the strong
covalent bonds between the atoms.
Molecules in phosphorus are attracted to one another by weak van der Waals’ forces. Only
very little heat is needed to separate the molecules.
c) The boiling point of an element depends on the strength of attractions between its particles.
The number of delocalized electrons for metallic bonding increases in the order Na < Mg <
Al.
Their atomic radii decrease in the order Na > Mg > Al. Both Mg and Al have multi-charged
ions. Thus, their ions have larger charge density and the electrostatic attractions with
electrons are stronger.
Hence the strength of metallic bonds and the boiling points of the elements increase from
Na to Al.
Suggested answers to in-text activities and unit-end exercises
Topic 12 Unit 41
10
© Jing Kung. All rights reserved.
New 21st Century Chemistry
13 —
14 The atomic radius of the elements is in the order F < O < S.
O and F belong to the same period. An atom of F has 1 proton more than an atom of O. The
shielding effect of the inner shell electrons is the same as both atoms have the same number of
inner shells.
Thus, compared with O, the effective nuclear charge felt by the outermost shell electrons of F
is greater. The electrons are pulled closer to the nucleus. So, the atomic radius of F is smaller
than that of O.
O and S belong to the same group. An atom of S has 1 more occupied electron shell than an
atom of O and the shielding effect of the inner shell electrons is greater.
Thus, compared with O, the effective nuclear charge felt by the outermost shell electrons of S
is smaller. The electrons are more loosely bound. So, the atomic radius of S is greater than that
of O.
15 a) i) Mg and Al belong to the same period. An atom of Al has 1 proton more than an atom of
Mg. The shielding effect of the inner shell electrons is the same as both atoms have the
same number of inner shells.
Thus, compared with Mg, the effective nuclear charge felt by the outermost shell
electrons of Al is greater. The electrons are pulled closer to the nucleus. So, the atomic
radius of Al is smaller than that of Mg.
ii) Mg and Ca belong to the same group. An atom of Ca has 1 more occupied electron
shell than an atom of Mg and the shielding effect of the inner shell electrons is greater.
Thus, compared with Mg, the effective nuclear charge felt by the outermost shell
electrons of Ca is smaller. The electrons are more loosely bound. So, the atomic radius
of Ca is greater than that of Mg.
b) The density of an element is its mass per unit volume.
The relative atomic mass of Al is greater than that of Mg.
The atomic radius and thus the atomic volume of Al is smaller than that of Mg.
Thus, the density of Al is greater than that of Mg.
16 a) Na, Mg and Al are all metals. They are good conductors of electricity due to the movement
of the mobile electrons.
Going from Na to Al, the number of delocalized electrons increases. There are more
mobile electrons. So, their electrical conductivities increase in the order Na < Mg < Al.
Suggested answers to in-text activities and unit-end exercises
Topic 12 Unit 41
11
© Jing Kung. All rights reserved.
New 21st Century Chemistry
b) In silicon, the four outermost shell electrons in each silicon atom are held in the four
covalent bonds which link the atom to four other silicon atoms.
Very few electrons gain enough energy to become delocalized. So, there are few
delocalized electrons and silicon is a poor electrical conductor.
17 a) P4(s) + 3O2(g)
P4O6(s)
b) i) The melting point of a compound depends on the strength of attractions between its
particles.
SO2 molecules are attracted to one another by weak van der Waals’ forces.
Only very little heat is needed to separate the molecules.
Thus, SO2 has a low melting point.
ii) Both MgO and Na2O are ionic compounds.
Mg2+ ion has a higher charge and smaller size than Na+ ion.
Hence the ionic bond between Mg2+ and O2– ions is stronger than that between Na+ and
O2– ions.
So, the melting point of MgO is higher than that of Na2O.
iii) SiO2 is a giant covalent compound with strong covalent bonds between the atoms.
P4O6 is a simple molecular compound with weak van der Waals’ forces between the
molecules.
Hence the melting point of SiO2 is much higher than that of P4O6.
c) i) An amphoteric compound can behave as an acid or as a base.
ii) Aluminium oxide can react with acids.
Al2O3(s) + 6H+(aq)
2Al3+(aq) + 3H2O(l)
Aluminium oxide also dissolves in sodium hydroxide solution to give a complex salt.
Al2O3(s) + 2OH–(aq) + 3H2O(l)
2[Al(OH)4]–(aq)
18 a) i) Na2O(s) + H2O(l)
2NaOH(aq)
ii) Ionic
b) i) P4O10(s) + 2H2O(l)
or
P4O10(s) + 6H2O(l)
4HPO3(aq)
4H3PO4(aq)
ii) Covalent
Suggested answers to in-text activities and unit-end exercises
Topic 12 Unit 41
12
© Jing Kung. All rights reserved.
New 21st Century Chemistry
c) The metallic character of the elements in Period 3 decreases across the period.
The oxides of the elements change from basic to acidic across the period.
d) i) CO2(g) + H2O(l)
H2CO3(aq)
ii) PbO(s) + 2HNO3(aq)
Pb(NO3)2(aq) + H2O(l)
+
or PbO(s) + 2H (aq)
Pb2+(aq) + H2O(l)
PbO(s) + 2NaOH(aq) + H2O(l)
Na2[Pb(OH)4](aq)
–
or PbO(s) + 2OH (aq) + H2O(l)
[Pb(OH)4]2–(aq)
19 a) Going across Period 3, there is a regular increase in the number of moles of oxygen atoms
that combines with one mole of atoms of each element. This number reaches a maximum
at Group VI.
b) The ions in solid magnesium oxide are not mobile.
c) i) Al2O3(s) + 6HCl(aq)
or
Al2O3(s) + 6H+(aq)
2AlCl3(aq) + 3H2O(l)
2Al3+(aq) + 3H2O(l)
ii) Al2O3(s) + 3H2O(l) + 6NaOH(aq)
2Na3Al(OH)6(aq)
d) Silicon has a giant covalent structure.
A lot of heat is needed to overcome the strong covalent bonds between the atoms.
Hence it has a high melting point.
e) P4O10 reacts with water to form an acidic solution.
P4O10(s) + 2H2O(l)
4HPO3(aq)
or
P4O10(s) + 6H2O(l)
4H3PO4(aq)
20 a) i) Covalent
ii) P
iii) Any one of the following:
• Phosphorous acid (H3PO3)
• Phosphoric acid (H3PO4)
• Metaphosphoric acid (HPO3)
Suggested answers to in-text activities and unit-end exercises
Topic 12 Unit 41
13
© Jing Kung. All rights reserved.
New 21st Century Chemistry
b) i) Giant covalent
ii) Si
iii) SiO2(s) + 2NaOH(aq)
Na2SiO3(aq) + H2O(l)
c) i) Ionic
ii) Na
iii) Na2O(s) + H2O(l)
2NaOH(aq)
21 a)
Element
Na
Mg
Al
Si
P
S
Cl
Formula of
common
oxide
Na2O
MgO
Al2O3
SiO2
P4O6 or
P4O10
SO2 or
SO3
Cl2O or
Cl2O7
Chemical
bonding of
oxide
ionic
Structure
of oxide
mainly ionic
mainly covalent
giant
covalent
giant ionic
simple molecular
b) i) Ga2O3 has an ionic structure with ionic bonding between Ga3+ and O2– ions.
As2O5 has a simple molecular structure with covalent bonding between As and O atoms
within the molecules. Weak van der Waals’ forces exist between the molecules.
ii) Ga2O3 is amphoteric and reacts with both HCl(aq) and NaOH(aq).
Ga2O3(s) + 6HCl(aq)
2GaCl3(aq) + 3H2O(l)
Ga2O3(s) + 2NaOH(aq) + 3H2O(l)
2Na[Ga(OH)4](aq)
As2O5 is acidic and reacts with NaOH(aq) but not HCl(aq).
As2O5(s) + 6NaOH(aq)
2Na3AsO4(aq) + 3H2O(l)
22 —
Suggested answers to in-text activities and unit-end exercises
Topic 12 Unit 41
14
© Jing Kung. All rights reserved.
New 21st Century Chemistry
23 The chemical formulae of common oxides of Period 3 elements are as follows:
Na2O, MgO, Al2O3, SiO2, P4O10, SO3, Cl2O7
Going across Period 3, there is a regular increase in the number of moles of oxygen atoms that
combines with one mole of atoms of each element. This number reaches a maximum at Group
VII.
The oxides of metals are mainly ionic while those of non-metals are mainly covalent.
Oxides of metals (Na, Mg and Al) have giant ionic structures. Oxide of silicon (SiO2) has a
giant covalent structure. Oxides of non-metals (P, S and Cl) have simple molecular structures.
In general, metals react with oxygen to form basic oxides. Most oxides formed from
non-metals are acidic oxides. Aluminium oxide is an amphoteric oxide.
MgO reacts with water to form an alkaline solution.
MgO(s) + H2O(l)
Mg(OH)2(s)
SO3 reacts with water to form an acidic solution.
SO3(g) + H2O(l)
H2SO4(aq)
Suggested answers to in-text activities and unit-end exercises
Topic 12 Unit 41
15
© Jing Kung. All rights reserved.








