The structure of calcite CaCO3
advertisement

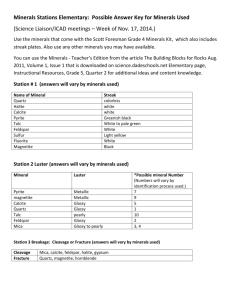
52 WHAT HAPPENS IF THE TETRAHEDRAL SHEETS AND OCTAHEDRAL SHEETS DON’T FIT TOGETHER? The serpentine group minerals with a general formula Mg3Si2O5(OH)4 has three forms : lizardite, chrysotile and antigorite. In each of these minerals there is a different way of coping with the tetrahedral-octahedral mismatch. In lizardite, the sheets are flat, but the tetrahedra have to rotate so that the apices can connect to the octahedral sheet, just as in kaolinite. In chrysotile, the tetrahedra are tilted and the layers are curved to accommodate the longer repeat length wuthin the octahedral layer. Continued growth of the curved layer results in a crystal in which the layers are rolled up as in a carpet. In antigorite, the tilting of the tetrahedra which leads to the curved layers is accompanied by a periodic switching of the direction of the tetrahedral layer. Another consequence of the misfit between the sheets is that the crystals are usually rather small, This is the case in clay minerals. 53 CLAY MINERALS Clay minerals are fine-grained (<0.002mm) sheet silicate minerals which form as a result of weathering of other silicates. The main clay minerals are: Kaolinite - Al2Si2O5(OH)4 O T 7Å O T Illite ~ K0.8Al2 (Al0.8Si3.2)(OH)2 i.e. essentially fine-grained muscovite T O T K+ 10 Å layer charge is not as high as in illite, so there are fewer cations in the interlayers, and also H2O can easily move in and out of the interlayers, causing the structure to expand and contract. The interlayer spacings can change from 10Å (when the clay is dry) to 15.2Å (when there are two layers on water molecules between the TOT layers). Smectites are therefore sometimes referred to as the swelling clays. T O T Smectite ~ Ca0.17(Al,Mg,Fe)2(Si,Al)4O10(OH)2 . nH2O Vermiculite ~ (Mg,Ca)0.3(Mg,Fe2+,Fe3+,Al)3(Si,Al)4O1 0(OH)2 Another group of swelling clays minerals is called vermiculite. It usually formed by the alteration of biotite by the oxidation of some Fe2= to Fe3+, thus lowering the negative charge in the biotite TOT layer. It has a higher layer charge than smectite. Chlorite: (Mg,Fe,Al)3(Si,Al)4O10(OH)2. (Mg,Fe,Al)3(OH)6 T O T T O T Na+. Ca2+.H 2O 10Å T O T Smectites form a large group of TOT layer minerals with both cations and water molecules between the layers. They may be either dioctahedral or trioctahedral and the net charge on the TOTlayers is the result of various substitutions in both tetrahedral and octahedral sheets (e.g. Al3+ for Si4+ in the tetrahedral sheets, or Mg2+ (or Fe2+) for Al3+ in the octahedral sheet.). The O 14Å T O T In clays chlorite can have a more complex composition than the larger chlrite crystals in rocks, and can be dioctahedral or trioctahedral. 54 FRAMEWORK SILICATES In the framework silicates all [SiO4] tetrahedra share their corners with others, generally forming rather open three-dimensional networks. If no ion substitutes for Si, the entire framework has the composition SiO2 and all valence bonds are satisfied. When Al substitutes for Si in the tetrahedra, interstitial cations are required to maintain charge balance. The openness of these framework structures results in rather large interstitial cation sites compared to those in the chain silicate minerals. Thus Na+ and Ca2+ will be considered as 'small' cations compared to K+, while ions such as Mg2+ are too small to play a role in these structures. The aluminosilicate framework minerals are by far the most abundant minerals in the Earth's crust, the feldspars making up about 65% by volume. In this course we will consider only the two most important groups of framweork silicates: the silica minerals and the feldspars. The silica minerals Silica, SiO2, occurs in a number of different forms in the Earth. Quartz, the most common crystalline polymorph is stable up to 857oC; tridymite is the stable form from 857oC to 1470oC, and then cristobalite from 1470oC up to the melting point at 1713oC. The high pressure forms of silica are coesite, stable in the deep crust of the Earth, and stishovite which is thought to be stable in the Earth's mantle. Stishovite has the rutile structure and is one of the very few known materials in which Si occurs in octahedral coordination with oxygen. The stability relationships of the SiO2 polymorphs are shown below. 55 The crystal structures of quartz, tridymite and cristobalite are related by reconstructive phase transitions, in other words, to convert one to another as the temperature increases requires breaking Si-O bonds and creating a new structure. This is a slow process, especially when the transitions take place on cooling, and both cristobalite and tridymite can be preserved metastably in volcanic rocks. Crystal structure of quartz. The crystal structure of quartz can be considered as being made up of 6-fold helices, spiralling around the c axis. The figure on the left shows one such helix. Looking along the helix axis, this gives the appearance of a hexagonal ring (right). The interlinking of such helices gives the full three-dimensional structure (bottom). 1 /3 2 /3 1 /3 0 2 /3 0 The structure shown above is that of high quartz which is stable above 573oC. Below that temperature the structure undergoes a displacive phase transition to low quartz. This transition is fast and is unavoidable, no matter how fast the quartz is cooled. No breaking of bonds is involved, just a twisting of the tetrahadra around the ‘joint’ which is the shared oxygen atom between tetrahedra. High quartz is hexagonal. Low quartz is trigonal. 56 Twin boun dary The structure of low quartz One consequence of the displacive transition is that there are two equivalent ways in which the structure can distort to trigonal symmetry. If different parts of the crystal distort in different ways, then the boundary between these regions is related by a simple symmetry operation and is called a twin boundary. The process of the formation of such boundaries is called transformation twinning and is a common phenomenon in many minerals. The structures of tridymite and cristobalite. These structures are related to one another, but are quite different from quartz. Both are made up of the same structural unit which is a layer of tetrahedra, with alternate tetrahedra pointing up and down. In both structures these layers are stacked one on the other. In tridymite they are stacked so that there is a two-layer repeat : ABABAB….. Tridymite is hexagonal. In cristobalite the layers are stacked with a three-layer repeat : ABCABC …. Cristobalite is cubic. 57 When these structures are cooled they should transform cristobalite tridymite quartz. As mentioned above, these are reconstructive transitions, and so very slow and difficult. If they do not occur, the crustobalite (and tridymite) will cool to lower temperatures (~ 200oC) and then distort by a displacive transition to low cristobalite and low tridymite, which have lower symmetry than the high forms. When tridymite and cristobalite are found in rocks they are always the low forms. These do not appear on the phase diagram because they are not thermodynamically stable. They are metastable, just as diamond is relative to graphite at room P,T. The feldspars Feldspars make up approximately 70% of the Earth’s crust, yet are structurally the most complex mineral group because of the many phase transitions which occur on cooling. In the feldspars some Al3+ substitutes for Si4+ in the framework, and charge balance is achieved by cations sited in the open spaces of the framework structure. The most common feldspars are the alkali feldspars, with compositions between KAlSi3O8 and NaAlSi3O8 , and the plagioclase feldspars with compositions between NaAlSi3O8 and CaAl2Si2O8. At high temperatures there is a complete solid solution between the alkali feldspar endmembers. At lower temperatures the solid solution exsolves (or unmixes) into regions that are Na-rich and regions which are K-rich. The result is that a single crystal of alkali feldspar contains an intergrowth at lower temperatures. This is called a perthitic texture. The scale of this perthitic texture depends on the cooling rate of the rock, but can usually only be seen by using a microscope. There is also a complete solid solution in the plagioclase feldspars at high temperatures. This solid solution is more complex because it involves the coupled substitution of Na for Ca and Al for Si: Na+ + Si4+ Ca2+ + Al3+ 58 At lower temperature this solid solution also breaks down, but the situation is more complex than alkali feldpars and the intergrowths can only be seen by electron microscopy. The structure of the feldspar minerals. The basic high temperature structure of the feldspars is shown below. (a) The basic building unit of the feldspar structure is the four-membered ring of tetrahedra with a pair of tetrahedra pointing up and a pair pointing down. Al and Si occupy these tetrahedra. (b) The four-fold rings are joined to form a layer in which the rings are related by mirror planes parallel to (010) and diads parallel to the b axis. Two sets of individual tetrahedra are distinguishable in this layer, and are labelled T1 and T2. The T1 tetrahedra are all related to one another by symmetry, as are the T2 tetrahedra. In the third dimension these layers are joined to each other by the apices. The K, Na and Ca cations occupy the large oval-shaped cavities between the rings. At lower temperatures the feldspar structures undergo: (i) displacive transitions, (ii) exsolution processes and (iii) Al,Si ordering processes. These processes are not independent and result in major complexities in the structures, which are not yet fully understood. The tendency for Al and Si atoms to become ordered in the tetrahedral sites at lower temperatures is a very general phenomenon. Understanding such Al,Si ordering transitions in minerals is a major topic in modern mineralogy because it has important consequences to the stability (thermodynamics) of minerals and rocks. It will be discussed in more detail in later Mineralogy courses. 59 CARBONATE MINERALS : CALCITE, DOLOMITE, ARAGONITE Trigonal and orthorhombic carbonates The geologically important carbonate minerals may be divided into two structural groups. The trigonal structure is adopted by carbonates of small cations while the orthorhombic structure is formed by carbonates of larger cations. The table below lists some members of each group with the ionic radius (in Å) of the cation. Trigonal (Calcite group) Orthorhombic (Aragonite group) Calcite CaCO3 (0.99) Magnesite MgCO3 (0.66) Siderite FeCO3 (0.74) Rhodocrosite MnCO3 (0.80) Smithsonite ZnCO3 (0.74) Dolomite CaMg(CO3) Ankerite Ca (Mg,Fe)(CO3) Aragonite CaCO3 (0.99) Witherite BaCO3 (1.34) Strontianite SrCO3 (1.13) Cerussite PbCO3 (1.20) The most important carbonate minerals are calcite, dolomite and aragonite. The structure of calcite CaCO3 CO3 Ca The structure of calcite, CaCO3, can be described in terms of a rhombohedron (essentially a cube which has been shortened along one of the triad axes). The Ca atoms have a face-centred distribution on this rhombohedron, and the triangular CO3 groups lie at the centres of each edge. This results in layers of CO3 groups lying normal to the c axis of calcite, with layers of Ca atoms lying between them . Calcite is the main constituent of limestone rocks. 60 The structure of dolomite Ca,Mg(CO3)2 The structure of dolomite is very similar to calcite but layers of Ca cations alternate with layers of Mg cations. The structure of aragonite The aragonite structure is preferred by carbonates when the cation radius is larger than about 1Å. Because Ca2+ lies on this boundary, it can fit into either structure. The structure also contains layers of triangular CO3 groups with the Ca atoms in between, but arrabged in a different way to that in calcite. The layers lie perpendicular to the c axis of the orthorhombic unit cell. The calcite-aragonite phase diagram. From this diagram we can see that aragonite is stable at high pressures and that calcite is the stable polymorph of CaCO3 at the earths surface. Aragonite does form in high pressure rocks, but also forms metastably under low pressure near-surface conditions. As well as forming the shells of some marine invertebrates, aragonite also forms in hotspring and cave deposits, and may also be directly precipitated from warm sea water. 61 When a mineral forms outside its stability field it must mean that the growth occurs under non-equilibrium conditions, and that there is some kinetic reason for its formation, i.e. it is easier to form aragonite than calcite under those particular conditions.