Silicates (several polymorphs)
advertisement

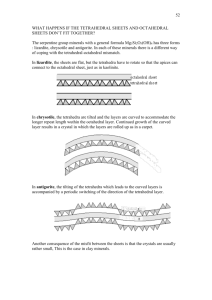
Silicates (several polymorphs) SiO2 Presented by Paul Sandlin • 3 principal crystalline forms - Quartz, tridymite, and cristobalite • Sluggish transformation, so high temp forms (cristobalite and tridymite) can exist metastably below their inversion temps • Each has low and high temp modification designated α and β respectively Alpha-Quartz Beta-Quartz Quartz • Quartz is most to a pure compound • Bachheimer (1980) found evidence for 1st-order transition from α-quartz to intermediate phase at 573°C and 2ndorder transition to β-quartz at 574°C - micro twinning upon cooling high quartz • Only minor atomic adjustments without breaking of Si-O bonds Quartz Occurrences • Common and abundant • Igneous, metamorphic, sedimentary, pegmatite veins, deposited on sea floor • Mechanically and chemically stable Orthorhombic-Tridymite Hexagonal Tridymite Tridymite monoclinic Tridymite • When pure quartz is heated, it bypasses tridymite and transforms directly to cristobalite at ~ 1050°C (Mosesman and Pitzer, (1941) - “Mineralizing agent” needed for tridymite formation • Several low-temp polymorphs • Ideally SiO2, but small amounts of Na and Al may be in solid solution • Stable from 870°C to 1470°C Tridymite occurrences • Typical occurrence is in acid volcanic rocks such as rhyolite, obsidian, trachyte, andesite and dacite. - Often found in cavities of such rocks • ? If it occurs magmatically (“metamorphic”) - pneumatolytic metamorphism • 6 months after Mt. Pelée eruptions Alpha- Cristobalite Beta-Cristobalite Cristobalite • Contains some Na and Al • Low cristobalite structure is tetragonal, whereas high cristobalite is isometric. • Stable from 1470°C to 1728°C (melting point) Cristobalite occurrences • Typically a mineral of volcanic rocks - may occur in cavities, often in association (metastable) with tridymite • Found in obsidian, rhyolite, trachyte, andesite, dacite, and olivine basalt. • Often a late product of crystallization • Due to the ability to occur as an unstable form outside equilibrium field, time of crystallization is difficult to pinpoint Coesite Coesite • Composed of four-membered rings of Si tetrahedra linked at corners to form chains parallel to c. • One Si-O-Si angle constrained to be 180° because this O1 site is located on a center of symmetry • Slight distortion occurs with pressure, and Si2-O2-Si2 angle decreasing from 142.7° to 136.4° at 5.19 GPa (Levien and Prewitt, 1981) Coesite occurrences • Recently discovered in sheared porous sandstones at Meteor Crater, Arizona • Granite and pumaceous tuff near the rim of the Rieskessel crater, Bavaria - developed by the shock wave generated by meteoritic impact Stishovite Stishovite • Prototype phase having octahedrally coordinated silicon • Structural properties at high pressure are highly sensitive to stress (Ross et al., 1990) • More compressible in the a direction than the c direction due to significant Si-Si repulsion across the shared edges of octahedra that form chains in the c direction (Ross et al., 1990) • At ambient conditions, O-O distance of 2.29Å is one of the shortest found in any oxide not containing hydrogen Stishovite occurrences • High pressure environments - meteoritic impacts