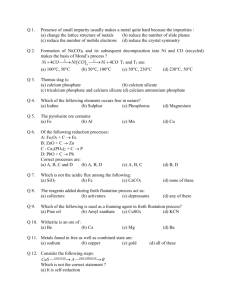
Ores
advertisement

Ores Ores A crude is any mixture of minerals in the form in which it occurs as a part of the earth's crust. An ore is solid crude containing a tillable constituent in such amounts as to constitute a promise of possible profit in extraction, treatment and sale. The valuable constituent of an ore is ordinarily called valuable mineral or often just mineral: the associated worthless material is called gangue. In some ores the mineral is in the chemical state in which it is desired by primary consumers, e.g. graphite, sulphur, asbestos, talc, garnet; in tact this is true of the majority of nonmetallic minerals. In metallic ores however, the valuable mineral is rarely the product desired by the consumer, and chemical treatment of such mineral is a necessary step in the process of benefication. In such cases the sale product is usually the result of concentration by the method of ore-dressing, followed by further concentration by the chemical methods of metallurgy. The valuable product of the ore-dressing treatment is called concentrate, the discarded waste is tailing. Concentrate is, in most cases, the feed to the metallurgical plant. If the metallurgical process is one in which separation is effected in a melt, the process is called smelting; if the separation is effected by differential or selective solution, the process is leaching or lixiviation; where the process is one of selective vaporization, it is called retorting. The valuable metal product of smelting is usually differently named according to the metal involved, e.g. blister (copper), base bullion (lead), pig (iron), etc.; the waste is slag. Metals occur in ores either in the native state (e.g., Au, Ag, Pt, Cu) or as salts or oxides (CuS, Fe2O3, PbCo3), etc. But no matter what chemical form, the metal or mineral is invariably associated with more or less — usually more — barren rock. The form in which the metal is required by the primary customers is a relatively pure substance. Hence a more or less extended process of purification, usually involving, in order, oredressing, metallurgical extraction, and chemical refining, intervenes between delivery of ore at the mine exit and delivery of the metal to the customers. One way in which the non-ferrous metals differ from iron is in the manner of their occurrence. Iron oxide occurs in large and comparatively pure deposits; the other metals and compounds from which metals are derived are scattered through large volumes of rock, such as limestone or quartz. Since it would be difficult and costly to smelt these large amounts of barren rock, metallurgists have recourse to concentration* or "ore dressing" by which the metals or metallic compounds are partially separated from the "gangue," or worthless material, before smelting. Gravity Methods of Ore Dressing. — The simplest method of ore dressing depends on the fact that in general the metallic compounds have a higher specific gravity than the gangue, and hence settle faster in a stream of water. Gold panning is the simplest illustration of the procedure. On a lager scale, it is carried on in jigs where the ore is placed on a screen and a pulsating stream of water forced through the screen, causing the lighter gangue to be washed out. Another form of gravity concentrator is the "table", consisting of a surface with longitudinal ridges, which is given a jerking end-to-end motion while a stream of water flows across, it laterally. By this means the heavy ore is shaken over the end while the gangue washes off the front. A crude is any mixture of minerals in the form in which it occurs as a part of the earth's crust. An ore is solid crude containing a tillable constituent in such amounts as to constitute a promise of possible profit in extraction, treatment and sale. The valuable constituent of an ore is ordinarily called valuable mineral or often just mineral: the associated worthless material is called gangue. In some ores the mineral is in the chemical state in which it is desired by primary consumers, e.g. graphite, sulphur, asbestos, talc, garnet; in tact this is true of the majority of nonmetallic minerals. In metallic ores however, the valuable mineral is rarely the product desired by the consumer, and chemical treatment of such mineral is a necessary step in the process of benefication. In such cases the sale product is usually the result of concentration by the method of ore-dressing, followed by further concentration by the chemical methods of metallurgy. The valuable product of the ore-dressing treatment is called concentrate, the discarded waste is tailing. Concentrate is, in most cases, the feed to the metallurgical plant. If the metallurgical process is one in which separation is effected in a melt, the process is called smelting; if the separation is effected by differential or selective solution, the process is leaching or lixiviation; where the process is one of selective vaporization, it is called retorting. The valuable metal product of smelting is usually differently named according to the metal involved, e.g. blister (copper), base bullion (lead), pig (iron), etc.; the waste is slag. Metals occur in ores either in the native state (e.g., Au, Ag, Pt, Cu) or as salts or oxides (CuS, Fe2O3, PbCo3), etc. But no matter what chemical form, the metal or mineral is invariably associated with more or less — usually more — barren rock. The form in which the metal is required by the primary customers is a relatively pure substance. Hence a more or less extended process of purification, usually involving, in order, oredressing, metallurgical extraction, and chemical refining, intervenes between delivery of ore at the mine exit and delivery of the metal to the customers. One way in which the non-ferrous metals differ from iron is in the manner of their occurrence. Iron oxide occurs in large and comparatively pure deposits; the other metals and compounds from which metals are derived are scattered through large volumes of rock, such as limestone or quartz. Since it would be difficult and costly to smelt these large amounts of barren rock, metallurgists have recourse to concentration* or "ore dressing" by which the metals or metallic compounds are partially separated from the "gangue," or worthless material, before smelting. Gravity Methods of Ore Dressing. — The simplest method of ore dressing depends on the fact that in general the metallic compounds have a higher specific gravity than the gangue, and hence settle faster in a stream of water. Gold panning is the simplest illustration of the procedure. On a lager scale, it is carried on in jigs where the ore is placed on a screen and a pulsating stream of water forced through the screen, causing the lighter gangue to be washed out. Another form of gravity concentrator is the "table", consisting of a surface with longitudinal ridges, which is given a jerking end-to-end motion while a stream of water flows across, it laterally. By this means the heavy ore is shaken over the end while the gangue washes off the front.