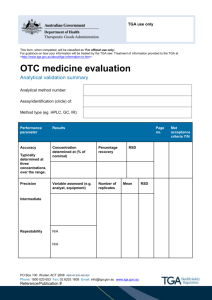
SCGT Quality Manual - Therapeutic Innovation Australia
advertisement

SCGT - QUALITY MANUAL SYDNEY CELL AND GENE THERAPY QS-SYS-P001 Version 2 Active: 5.10.11 Documents not printed on controlled paper are valid for the print date only CONFIDNETIAL Page 1 of 23 Author: R Makin SCGT - QUALITY MANUAL TABLE OF CONTENTS SECTION 1 INTRODUCTION ......................................................................................... SECTION 2 THE QUALITY SYSTEM ........................................................................... SECTION 3 ORGANISATION CHART .......................................................................... SECTION 4 RESPONSIBILITIES AND ROLES ........................................................... SECTION 5 DOCUMENT CONTROL ............................................................................ SECTION 6 RECORDS ..................................................................................................... SECTION 7 PERSONNEL AND TRAINING ................................................................. SECTION 8 BUILDING AND FACILITIES ................................................................... SECTION 9 EQUIPMENT ................................................................................................ SECTION 10 QUALIFICATION AND VALIDATION ................................................... SECTION 11 CONTROL OF MATERIAL ....................................................................... SECTION 12 DONOR SELECTION, DONATION AND TESTING ............................. SECTION 13 PROCESS CONTROL ................................................................................. SECTION 14 STORAGE, PACKAGING AND TRANSPORT ....................................... SECTION 15 AUDITS .......................................................................................................... SECTION 16 NON-CONFORMANCE MANAGEMENT ............................................... SECTION 17 CORRECTIVE ACTION/ PREVENTIVE ACTION ............................... SECTION 18 QUALITY IMPROVEMENT ..................................................................... SECTION 19 MANAGEMENT REVIEW ......................................................................... SECTION 20 ETHICS .......................................................................................................... SECTION 21 COMPLAINTS .............................................................................................. SECTION 22 EXTERNAL USERS ..................................................................................... APPENDIX 1 CHANGES TO PREVIOUS VERSION...................................................... APPENDIX 2 RELEVANT STANDARDS AND GUIDELINES...................................... QS-SYS-P001 Version 2 Active: 5.10.11 Documents not printed on controlled paper are valid for the print date only CONFIDNETIAL Page 2 of 23 Author: R Makin SCGT - QUALITY MANUAL 1 Introduction Sydney Centre for Cell and Gene Therapies, hereafter abbreviated as SCGT, is based in one of the largest healthcare and research precincts in the Southern hemisphere which encompasses two Area Health Services and three Medical Research Institutes. The SCGT provides a seamless integration of the extensive knowledge, expertise and facilities of the following organisations: The Children’s Hospital at Westmead (CHW) Children’s Medical Research Institute (CMRI) Westmead Hospital (WH) Westmead Millennium Institute (WMI) Kids Research Institute (KRI) The SCGT has the largest clinical and research experience in cell and gene-based therapeutics in Australia, with capacity to support third party investigators through access to our template for success and in house facilities. These facilities include fully commissioned state of the art, aseptic and aseptic/ bio-contained gene and cellular therapeutics manufacturing suites. The services and research programs currently undertaken by SCGT include: 2 The largest blood and marrow stem cell transplantation laboratory in NSW The first Clinical Islet Transplant Unit in Australia and only facility in NSW to have performed more than 5 islet transplants for patients with Type I diabetes. Research program of Xenotransplantation (pig organs into humans) Active clinical trials involving adoptive immunotherapy of T cells for opportunistic infection. The largest and most productive gene therapy research program in Australia. The Quality System The quality system applies to all processes and functions of SCGT that require quality management control. This includes but is not limited to activities associated with all stages of the collection, processing, testing, storage, and release of product, and the training and development of SCGT personnel. Compliance with the Quality Policies and Procedures of SCGT is mandatory. These quality policies also apply to all manufacturing processes that are carried out in the facility by external staff. It is the responsibility of SCGT management to ensure that all external staff members complete the requisite training in the SCGT policies and procedures before commencing any processing in the facility. This Quality Manual (QM) describes the quality system of SCGT including the organisational and management structure. This QM provides “a map” of the policies and procedures covering all of the manufacturing functions of the SCGT. The objectives and policy statements contained within the quality manual are implemented through procedures that describe the operation of the quality system components. This quality manual is a controlled document and is distributed to authorised holders in accordance with SCGT document control procedures. QS-SYS-P001 Version 2 Active: 5.10.11 Documents not printed on controlled paper are valid for the print date only CONFIDNETIAL Page 3 of 23 Author: R Makin SCGT - QUALITY MANUAL The SCGT has developed and implemented an effective quality system that is based on the principles of good manufacturing practice and ensures that wherever possible all products and services meet or exceed the best practice standards for quality, efficacy and safety. The SCGT operates in accordance with all applicable Commonwealth and State / Territory policy frameworks and laws, particularly those pertaining to the regulation of human cellular therapies. The manufacture of all cell and gene therapies by SCGT is governed by the following applicable standards: Therapeutic Goods (Manufacturing Principles) Determination No 1. Australian Code of GMP - Human Blood and Blood Components, Human Tissues and Human Cellular Therapies TGA. Note for Guidance on Good Clinical Practice TGA AS ISO 15189 Medical Laboratories – Particular requirements for quality and compliance AS ISO 15189 Field Application Document Medical Testing Supplementary requirements for accreditation (FAD) FACT-JACIE International Standards for Cellular Therapy Product Collection, Processing and Administration FACT. Requirements for Procedures related to the Collection, Processing, Storage and Issue of Human Hemopoietic Progenitor Cells NPAAC. Quality Policy Statement SCGT is committed to excellence in research and manufacture of gene and cellular therapies. Our mission is to be a world leader in this field. Our quality goal is to meet all relevant TGA regulations and FACT accreditation standards in order to fulfil and exceed the missions of our collaborative partners (customers). The SCGT steering committee supports this policy and each staff member is responsible for implementing this policy in every area of work. Employees are encouraged to look for improvement opportunities to enhance the performance of the Quality System. It is the responsibility of the Facility Director with the assistance of the Quality Manager to ensure that the quality policy is implemented and complied with by all SCGT staff. At present this position is shared by the Unit Directors. Arbitration of any conflicting ideas is resolved via the Steering Committee under the direction of the Chair. The Directors along with the Quality Manager and the Steering Committee will routinely set measurable quality objectives to be met by all groups. The Quality System is reviewed for effectiveness at least annually in a formal Management Review meeting. Vision The vision of the SCGT is to be a world leader in health and medical research based on globally competitive research and health outcomes, which directly benefit the community and are linked to the development of a vibrant regional biotechnology and health services industry. Values S Scientific integrity C Cellular and genetic therapies allowing care for patients beyond the boundaries of established medicine G GMP Compliance T Translating high quality research into clinical outcomes for patients QS-SYS-P001 Version 2 Active: 5.10.11 Documents not printed on controlled paper are valid for the print date only CONFIDNETIAL Page 4 of 23 Author: R Makin SCGT - QUALITY MANUAL Chair, Westmead Research Hub Steering Committee: Professor Jeremy Chapman Signature _______________________ Date __________ Director, BMT and Cellular Therapy: Professor David Gottlieb Signature _______________________ Date __________ Head, Gene Therapy Research Unit: Professor Ian Alexander Signature _______________________ Date __________ Director, Westmead Islets Transplantation: Professor Philip O’Connell Signature _______________________ Date __________ References: QS-SYS-P002 (Management Review) QS-SYS-P001 Version 2 Active: 5.10.11 Documents not printed on controlled paper are valid for the print date only CONFIDNETIAL Page 5 of 23 Author: R Makin SCGT - QUALITY MANUAL 3 Organisational Chart – Structure Key Westmead Research Hub Executive Westmead Research Hub is BLUE External users are Orange See QS-SYS-P006 (Organisational Chart – Personnel) for staff details Hospital Maintenance, engineering, IT etc BMT Network Quality Manager Centre Administrator Director of BMT and Cellular Therapy Unbroken lines represent reporting relationships. Steering Committee Director of Westmead Islets Transplantation Dotted lines represent interactions Director of Gene Therapy Professor Director Quality Manager Deputy Director of BMT and Cellular Therapy Production Manager TCR Production Manager BMT Production Manager MGMT Production Manager WIT Production Manager Quality Officer Operations Managers BMT Scientist’s x3 Scientist Scientist QS-SYS-P001 Version 2 Active: 5.10.11 Documents not printed on controlled paper are valid for the print date only CONFIDNETIAL Non core Scientist s x 5 Scientist Page 6 of 23 Author: R Makin Quality Officer Scientist SCGT - QUALITY MANUAL 4 Roles and Responsibilities Facility Director The Facility Director is ultimately responsible for ensuring the quality of product and compliance of SCGT with its own quality system and relevant international and national requirements. Directors Director Gene and Cell Medicine Facility– Prof Ian Alexander Director of the Sydney Cellular Therapies Laboratory – Prof David Gottlieb Director of the Westmead Islets Transplantation – Prof Philip O’Connell Chair, Steering Committee Prof Jeremy Chapman Facility Co-ordinator Margot Latham Production Nominees Cancer Gene Therapy Clinical Trial Leader – Dr Belinda Kramer Islet Laboratory Production Manager – Dr Wayne Hawthorne BMT Senior Scientist –Vicki Antonenas T-Cell Clinical Research – Dr Leighton Clancy Quality Manager Richard Makin As defined by the SCGT organisation chart the quality assurance nominee (i.e. Quality Manager) is independent of the reporting lines of the manufacture of the product but has appropriate authority to ensure the quality of the product. The Quality Manager reports directly to the Steering Committee. The production nominee or manager (i.e. the scientific managers of the units) has the necessary authority to control the manufacture of the product. The production nominee reports directly to the Directors of the units. In the event that there is conflict between the production activities and the quality policy it is the responsibility of the Steering Committee to resolve the issue and ensure appropriate records of the decision are maintained. Deputies Position Facility Director Unit Director Chair of Steering Committee Production Manager Quality Manager Deputy Director of another unit Production Manager of the Unit Nominated Unit Operations Manager or Scientist Quality Officer References See QS-SYS-X001 to QS-SYS-X008 Position Descriptions QS-SYS-P001 Version 2 Active: 5.10.11 Documents not printed on controlled paper are valid for the print date only Page 7 of 23 Author: R Makin SCGT - QUALITY MANUAL 5 Document control Policies, procedures, forms, templates, information sheets of the SCGT are managed via Q-Pulse software following the document control procedures. All SCGT quality and facility policies and procedures reside in the SCGT Q-Pulse system. The production documentation for the Westmead Islets Transplantation, MGMT Trial and T-Cell Clinical Research also reside in the SCGT Q-Pulse system. The production documents of the BMT laboratory reside in the BMT Network’s quality system. An MOU has been signed by both parties to allow the Quality Managers of the BMT Network and SCGT to review each other’s documentation to ensure consistency. External standards and guidelines are entered into Q-Pulse as external documents. Staff should ensure these have not been superseded prior to referring to them. The units are obliged to comply with the policies and procedures of the hospitals and research institutes in which they reside. These are reviewed via the intranet and internet. MOUs are established with the organisations (e.g. donor co-ordinators) controlling additional documentation which mat effect the units to ensure that the documents are reviewed periodically and that only current versions are available for use. References QS-SYS-S003 (Document Control) QS-SYS-S010 (Document control – Q-Pulse) SCGT-BMT Network MOU 6 Records Records provide evidence that what was required to be done was actually done. Records provide the evidence that the quality system is implemented. Any record that has a bearing on the quality of a product manufactured at and/or supplied by SCGT, and which is necessary to ensure traceability or to demonstrate that specific actions were taken in the event of challenges by outside parties, must be retained for a minimum of 20 years according to the code of GMP. This period of retention may be greater if specified by other parties such as another regulatory body. This includes (but not limited to) records for the following: Critical materiel Purchase orders Donor/patient Inspection and testing Collection and processing Equipment checks, calibrations, cleaning Equipment and facility servicing and maintenance Packaging and labelling Product release Quality Control Training Audit Validations QS-SYS-P001 Version 2 Active: 5.10.11 Documents not printed on controlled paper are valid for the print date only Page 8 of 23 Author: R Makin SCGT - QUALITY MANUAL Staff are responsible for: Ensuring that procedures incorporate requirements to capture and record data and information; Ensuring that hand written entries are clear, legible and any amendments are corrected to allow legibility of the original data; Ensuring that forms used to record data are designed to allow clear entry of data, meet task traceability requirements and are part of the document control system; and Developing procedures for collation and storage of records so that they are secure, readily retrievable, are protected from deterioration, and are batched prior to archiving. The Quality Manager is responsible for developing and implementing procedures for storage of archived records in a form which provides adequate security and confidentiality, and prevents deterioration for at least the statutory 20 year period and ensures that archived records are readily accessible. Paper records are protected from environmental and pest damage. Locked cabinets are used to secure records from unauthorised access. Records of products not released are stamped with “Quarantined” to distinguish them from those that conform to specification. Corrective actions to products which do not meet the required specification must be adequately detailed to demonstrate the resolution of the non-conformance. This detail is recorded in QPulse as a CAPA. Recorded information includes: Traceability of all critical steps in the procedure; The identity of the person making the record; Date, and where relevant the time of each step performed; All original information (e.g. observations and calculations); Equipment used; Identity of the person authorising steps and checking transcriptions; Quality control results; Inspection check results; Product release authorisation: and Any other information specified in the SOP, other contractual documents or relevant statutory regulations. Computer records SCGT records are primarily paper-based however records may also be in the form of microfilm, microfiche, computer files, or CD-ROM. If computer systems are used to record critical process related information then the computer system must be properly validated and data must be secure. Data should not be able to be changed, if changes are made then there must be an audit trail provided by the system. Records may be scanned and stored electronically; however, it is ensured that all original information is included in the scan. Data and information relating to the services and products supplied by SCGT is captured and recorded, such that evidence is available to demonstrate: Achievement of quality requirements; Fulfilment of contractual requirements; Fulfilment of regulatory requirements; Effective operation of systems and procedures; Maintenance of inspection and test equipment; and A complete history of the product. QS-SYS-P001 Version 2 Active: 5.10.11 Documents not printed on controlled paper are valid for the print date only Page 9 of 23 Author: R Makin SCGT - QUALITY MANUAL When purchasing or upgrading a computer system it is important that the system is compatible with current systems. This is assessed prior to a commitment to purchase is made. The data saved from the old computer systems must be able to be accessed for the required retention time period. It is the policy of SCGT that vendors have an established quality system e.g. ISO 9001 or an equivalent standard. A certificate is requested of the vendor to demonstrate compliance. References: QS-SYS-S005 (Records) 7 Personnel and Training Supervision The Unit Directors are responsible for ensuring that there are sufficient staff resources with relevant qualifications, experience and competence across the SCGT to ensure that the quality system is maintained and consistently high quality products are manufactured. This is achieved by: A training protocol for all staff which includes orientation training, training in assigned tasks, ongoing and refresher training and periodic competency assessments; Records maintained of SOPs being read by all relevant staff. The acknowledgement of updates and changes to documents is recorded via Q-Pulse; Trainees being signed off by the trainer to demonstrate competencies in assigned tasks; Maintaining a register of staff signatures; Training in the safety aspects of the laboratory (e.g. fire, MSDS, personnel hygiene); Staff being trained in the product requirements e.g. cGMP; Staff receiving training in the SCGT quality system; Access to internal continuing education activities i.e. journal club; Access to external continuing education activities e.g. workshops Access to relevant texts, journals and internet; and Involvement in relevant professional societies e.g. ISCT. Supervisory staff have adequate experience in manufacturing the products they oversee and have a thorough knowledge of the requirements of cGMP, FACT and ISO 15189 to ensure products consistently meet the specified requirements. Supervisory staff have adequate authority to discharge their responsibilities, where relevant. Deputies for key tasks are assigned for all sections. There is currently no requirement for the facilities to run routinely out-of-hours and have dedicated outof-hours staff. If this case arises it is SCGT policy that these staff must have regular contact with supervisory staff and work normal hours at least one day per month for personal / professional development. Where any part of the manufacture of a product is not performed on-site (e.g. donor collection) a contract with the provider is set up to ensure that the specification requirements are met and staff are adequately trained with detailed training records maintained. The responsibilities of individual staff are defined in the position descriptions along with the deputies for key tasks. Roles have been clearly defined so that there is no conflict of interest or overlap in responsibilities which may hinder the production of a quality product. QS-SYS-P001 Version 2 Active: 5.10.11 Documents not printed on controlled paper are valid for the print date only Page 10 of 23 Author: R Makin SCGT - QUALITY MANUAL References: QS-SYS-P005 (Training overview) QS-SYS-X001 – QS-SYS-X008 Position Descriptions 8 Facilities Design SCGT consists of four clean room facilities which have been designed to meet the production requirements, the safety and comforts of its occupants and the requirements of cGMP. Regular cleaning, sanitising, monitoring and maintenance are performed to ensure the quality of the products being manufactured. Access The facilities are secure from unauthorised access via swipe card entry. Detail of access control is provided by the Security SOPs. A list of authorised users is maintained for each of the facilities. Monitoring Temperature and humidity are continuously monitored via the building maintenance systems. These building maintenance systems are alarmed centrally. Where temperature and humidity levels exceed the acceptable limits corrective action is recorded and a decision on the use of the product is made. The acceptance or rejection of the product is made by the Director and Production Manager in consultation with relevant clinicians. Environmental monitoring (microbial, particulate and viable air sampling) is important in ensuring an aseptic manufacturing environment for products. The environmental monitoring SOP details the frequency and extent of environmental monitoring in the facilities. Cleaning Cleaning of the facilities is performed as detailed in the Cleaning SOP. This is made available to all laboratory and cleaning staff. The adequacy of the use of the protocol is assessed via auditing and environmental monitoring. Training of the cleaners in cGMP building and facility requirements is provided. Storage Purpose built storage facilities are implemented across SCGT. These allow for the suitable storage conditions for product and segregation of non-conforming product and material. These storage facilities are continuously monitored by centrally alarmed systems and are routinely environmentally monitored. Safety Staff are required to follow the OHS policies of the hospitals and research institutes. These are available via the intranet of each hospital. Regular training sessions (e.g. fire, hand cleaning) are conducted. All chemicals stored in the facilities are listed in the Chem Alert MSDS database as per the Westmead Hospital and Kid Research Institute requirements. Chemical spill kits are available which are relevant to the chemicals stored in the facility. Eyewash, hand wash and safety shower facilities are readily available. References: RE-SYS-S001 (Environmental Monitoring) RE-SYS-S003 (Cleaning cleanrooms and associated rooms) RE-SCT-S002 (SCTL Access and Security) RE-SCT-S004 (Monitoring of SCTL) RE-KRI-S010 (GCMF Access and Security) QS-SYS-P001 Version 2 Active: 5.10.11 Documents not printed on controlled paper are valid for the print date only Page 11 of 23 Author: R Makin SCGT - QUALITY MANUAL 9 Equipment All equipment is suitably procured and / or designed and built to ensure the reliable manufacture of product(s) to the specified requirements. Equipment is regularly checked, calibrated and serviced to ensure reproducibility and consistency in product manufacture. These steps ensure that equipment is fit for the purpose. Qualification and validation All equipment (including computer software used in critical control steps) is commissioned prior to use according to the Validation Master Plan. A validation report is completed as a record of commissioning. In addition any major change to the equipment (new software) is validated. Equipment (including portable equipment) is used in accordance with the manufacturer’s recommendations unless the change has been validated. All operational checks defined by the manufacturer or equipment SOP are completed and recorded. Equipment which was in use prior to the implementation of the quality system and has not been formally commissioned must have extensive quality control and quality assurance data accumulated and recorded to demonstrate its reliability and reproducibility. Existing equipment may undergo retrospective validation if the process it carries out is a critical step. Preventative Maintenance All equipment is included on the preventative maintenance schedule. Scheduled maintenance /shutdown maintenance/servicing are conducted externally following established contracts and records of the service maintained. Short term preventative maintenance is performed in-house, where possible or where an external contractor is not available. This is performed in accordance with the maintenance/servicing SOP. Training of staff in preventative maintenance is provided during initial training. Calibration, checks and verification Equipment is calibrated or checked according to the manufacturer’s recommendations or specified standards, which ever calibration/ check interval is shorter. Where equipment calibration/ check requirements are listed in Section 4 - Equipment Calibration table of the NATA Medical Testing FAD the timeframes and methods are followed. Where a calibration to a reference material / standard has been performed a certificate for this reference material / standard is obtained. The provider of the service should be NATA accredited and the certificate provided should include the NATA logo and accreditation number. If this is absent, the accreditation status should be checked on the NATA website (www.nata.com.au ) and the service contract reviewed. Equipment which does not meet the manufacturer’s recommendations or specified standards must be taken out of service and clearly labelled as ‘OUT OF SERVICE’. Following servicing and prior to use the piece of equipment must be verified to be suitable for its intended use. The verification must be recorded on the verification worksheet. Equipment must also to be verified to be suitable for use when it has been moved or major repair work performed. Revalidation may be required. The protocol for calibration/ checking of equipment must be included in the equipment SOPs. The minimum information required is: Frequency of calibration and/or check; Method used; Acceptable limits (define by the purpose of the equipment); and Action to be taken to non-conforming equipment. Specific monitoring requirements are included in the specific equipments SOPs. QS-SYS-P001 Version 2 Active: 5.10.11 Documents not printed on controlled paper are valid for the print date only Page 12 of 23 Author: R Makin SCGT - QUALITY MANUAL Equipment records (e.g. temperature charts) must include the actual results and the acceptable limits. Action must be taken and recorded to non-conforming results. References: RE-SYS-S018 (Validation Master Plan) QS-SYS-P008 (Contract Management) 10 Qualification and Validation SCGT facilities, equipment and processes must be designed, installed, operated to meet the product specifications and to consistently manufacture high quality products. Processes include critical computer systems as well as facilities and equipment. Prior to the introduction of a new process, staff initiate a change control to document and cost the planned process. The change control process allows all responsible managers to review the proposed new process. The change control process includes a risk assessment which looks at effect on donor / patient safety and costs of the new or changed process or equipment. If approved to proceed the new process will be included in the validation schedule. Existing processes are also considered for requalification and revalidation in the Validation Master Plan. The Validation Master Plan includes; Scope of validation operations; Responsibilities, resourcing, costs; Product types or description (could cross-reference similar validations); Critical considerations; Systems, or equipment or test processes to be validated; Acceptance criteria; Required SOPs; Change control; Retraining of personnel Authorisation Validation status of each process or piece of equipment Revalidation requirements Once a validation protocol and/or design specification has been established the validation is conducted and documented. The process of Installation Qualification (IQ), Operational Qualification (OQ) Performance Qualification (PQ) is followed. It is possible that some validations may be assisted/ performed externally. It is, however, the responsibility of SCGT that these validations are adequately detailed to demonstrate that product specifications will be met. Change control All changes to facilities, equipment, material and processes, including test methods which will affect the manufactured product must be reviewed, validated and authorised by the relevant supervising staff (e.g. Unit Director, Production Manager, and Quality Manager). The degree of validation will be determined by the extent of the change. All significant changes must be notified to relevant staff and client/ clinician. Detail of the required review, authorisation, re-qualification and notification is included in the change control and validation procedures. QS-SYS-P001 Version 2 Active: 5.10.11 Documents not printed on controlled paper are valid for the print date only Page 13 of 23 Author: R Makin SCGT - QUALITY MANUAL The relationship between change control and IQ/OQ/PQ Change Control Preapproval Pre-approval of changes to facility, systems or equipment is required via the change control system. Complete TE-SYS-F008 (Change control form) Design Qualification (DQ) / User Requirement Specification (URS) DQ- Check that the facilities, system and equipment have been designed in accordance with GMP e.g. equipment is a certain brand, has a service contract, displays certain data. Installation Qualification Operational Qualification Performance Qualification Change Control Verification of the study and final approval URS – Brief provided to prospective manufacturers or service providers outlining the unit’s requirements. IQ - The documented verification that the facilities, systems and equipment as installed or modified, comply with the approved design and manufacturer’s recommendations. This information is often provided by the manufacturer, however, a check should be made against the manufacturers’ requirements and purchase specifications. OQ - The documented verification that the facilities, systems and equipment, as installed or modified, perform as intended throughout the anticipated operating ranges e.g. running replicates of QC. Complete: TS-SYS-S012 (Validation Protocol Template) TS-SYS-F020 (Validation Report Template) PQ – Assessment of the facility, systems and equipment to perform its intended task reproducibly and effectively following relevant SOPs and product requirements. Complete: TS-SYS-S012 (Validation Protocol Template) TS-SYS-F020 (Validation Report Template) Change control – formal system for the notification and authorisation of change including validations. Complete CCF. References: RE-SYS-S018 (Validation Master Plan) QS-SYS-P004 (Change control) TE-SYS-S002 (Validation Protocol Template) TE-SYS-F020 (Validation Report Template) PIC/S Recommendations on validation master plan, Installation and operation qualification, Non-sterile process, Cleaning Validation QS-SYS-P001 Version 2 Active: 5.10.11 Documents not printed on controlled paper are valid for the print date only Page 14 of 23 Author: R Makin SCGT - QUALITY MANUAL 11 Control of Material All critical material is verified to meet required specifications prior to use in manufacturing of product. Critical material is defined by cGMP as all components, materials or supplies which could have a direct negative impact on the quality of the end product or a direct negative impact on the patient. Chemicals used in the manufacture of products are evaluated prior to use against relevant material specifications. Each chemical/ ingredient has a material specification sheet. Vendors of critical materials have been assessed via the Vendor Approval process. Critical materials are purchased only from approved vendors. Assessment of material Validation against Material Specification Sheet Production Manager to co-ordinate Nonconforming material Director/ Production Manager to co-ordinate Quarantine Found to not comply during processing or expired Material Receipt Material receipt record Conforming Material Discard procedure Potential for unsafe product TGA Discard No potential for unsafe product Validation Ready for production Product Recall The product recall procedure is based on the Australian Uniform Recall Procedure for Therapeutic Goods. The TGA will be notified if product does not conform to requirements. Storage Conforming and quarantined material are clearly separated. Quarantined product is stored in a lockable storage area / container. Material is stored as per the manufacturer’s recommendations. Storage facilities are monitored for temperature and humidity, where necessary for maintaining the integrity of the material. Sterile solutions are labelled as ‘sterile for therapeutic use’. Where sterility is tested externally records of the sterility testing are maintained. Where sterile packs are used the integrity of the pack is checked prior to use. It is the policy of SCGT that expired material is not to be used in the manufacture of a product unless comprehensive validation of the extension to the expiry date is maintained. Expired material is quarantined. QS-SYS-P001 Version 2 Active: 5.10.11 Documents not printed on controlled paper are valid for the print date only Page 15 of 23 Author: R Makin SCGT - QUALITY MANUAL Transport of material The transport requirements for material are specified in the Material Specification Sheet. Where material is transported within the facility or to another facility the specifications are followed. Labelling All material is uniquely identified by the product name and batch / lot number. The status of the material is also included e.g. received, released. It is SCGT policy that only specifically designed labels (e.g. adhesive, format) be used for products i.e. Meet TGO labelling requirements. Label batches are checked prior to use to ensure they are not duplicates or do not include the incorrect information. Labels are only produced by authorised personnel. When not in use labels are securely stored in a locked Production Managers office in mater folders. Purchasing and Sub-contracting A list of suppliers of material and contractors is maintained for each of the units. Suppliers are assessed against the following criteria prior to entering into a formal contract: There supplies meet product and material specifications; They are reliable and consistent; They provide customer support; Where relevant, they have a suitable quality system and have evidence of such a system (ISO 9001 certificate, AS ISO15189 accreditation certificate or TGA Licence number); and They are able to provide adequate service records. Once these criteria are met contract arrangements are made with the supplier including specification details and the limits of rejection. The material is assessed on receipt and any deviations from the specifications are fed back to the supplier via the Production Manager. References: www.tga.gov.au RE-SYS-S009 (Material Management) QS-SYS-S008 (Product Recall) RE-SYS-S019 (Selection, Qualification and monitoring of vendors) RE-SYS-S017 (Procurement) 12 Donor Selection, Donation and Testing Donor procedures are documented and implemented by the following associated organisations: Sydney Cellular Therapies Laboratory Westmead Bone Marrow Transplant Laboratory BMT Network NSW Westmead Islet Transplantation Donor Transplant Co-ordinator Gene and Cell Medicine Facility MGMT Trial CHW Oncology Contracts are in place with each of the organisations which require their procedures polices and documents to be compliant cGMP and relevant privacy legislation. References: DO-WIT-S001 (Islet Transplantation) QS-SYS-P001 Version 2 Active: 5.10.11 Documents not printed on controlled paper are valid for the print date only Page 16 of 23 Author: R Makin SCGT - QUALITY MANUAL 13 Process control The quality of the products is determined by the material, procedures and controls in place, capability of the equipment used, the competency of staff to manufacture the product to the specifications and the consistent maintenance of the equipment and facilities. These fundamental components are monitored and maintained by: Regular internal audits; Sampling of product (to determine how to do this); Monitoring of humidity and temperature; Environmental testing; Staff training and competency assessment; and Equipment checking, calibration, servicing and maintenance. Quality control Quality controls are used to demonstrate that processes are under adequate control. Quality controls are performed in compliance with the documented procedures and results recorded. Any non-conformance in quality control (QC) results is recorded along with the corrective taken. The Production Manager is informed where the QC results indicate a problem with the product. The Production Manager will periodically perform a long term review of QC results and process records to determine trends. Issues with QC results and processes are discussed with all relevant staff via Unit meetings. Any significant issues are raised at the Quality and Steering Committee Meetings. Product release Products are signed out for release by the Unit Director /Production Manager. The criteria listed in the Product Release Specification must be fulfilled prior to release of products. Products which do not meet these criteria are place in quarantine. A review of the batch documentation is performed by the Quality Manager. Testing of product Product testing is performed in compliance with the product specifications. Products are tested using external laboratories (e.g. ARCBS), internally (e.g. Research Laboratory) and associated pathology laboratories (e.g. Westmead Hospital Microbiology Department). References: PR-SYS-S001 (Review of Batch Documentation) QS-SYS- P007 (Product Release) QS-SYS-P009 (CAPA Policy) QS-SYS-S008 (Product Recall) 14 Storage packaging and transport Storage Storage conditions of the facilities are monitored via building maintenance systems which are alarmed centrally and /or externally depending on the parameter being monitored. Products ready for release are clearly segregated from quarantined product. Quarantine product is placed in a secured store which is clearly labelled as ‘Quarantined’. The product itself is also labelled as ‘Quarantined’. A log of quarantined product is maintained which details the production donation number and the reason for its quarantined status. Quarantined product can only be removed by the Production Manager. QS-SYS-P001 Version 2 Active: 5.10.11 Documents not printed on controlled paper are valid for the print date only Page 17 of 23 Author: R Makin SCGT - QUALITY MANUAL If the quarantined product is subsequently transported the ‘Quarantined’ label is checked to be firmly attached. The transport container is also labelled ‘Quarantined’. Discard The disposal procedure for each product is defined in the disposal SOP. All products to be disposed of is labelled as such and stored in the quarantine area. The product is then disposed of in the timeframe stated in the disposal SOP. A record of the donation product number, date of disposal, reason for disposal, how it was disposed of, and the staff member disposing of the product is maintained on the log of discarded product. Labelling Product labels are used to identify the status of the product. A label is attached to the product and the production records to enable reconciliation. Any product with uncertain status is segregated and accompanied by a record detailing the reason for segregation and any investigation / corrective action. The particulars on product labels follow the Therapeutic Goods Order No.69. Product labels are placed in a locked store when not in use. Packaging Product to be transported is packaged and labelled using specified packaging instructions. The minimum information allowable on the transport container is: The address and contact name of the site of origin of the product and its destination; and The contents of the container. A consignment note is accompanied with each container. The minimum allowable information on the consignment note is: SCGT plus the name of unit (e.g. Cancer Gene Therapy); Name of the receiving site; Container number; Name and status of product(s); Donation number of product(s); Total number of product(s); Date/time of dispatch; and Signature of staff member authorising the dispatch. When the product is received by the recipient the date and time is also recorded. Transport SCGT is responsible for the transport conditions of the product prior to receipt of the product by the recipient. If an external courier service is used they are informed of the transport requirements. Acknowledgement of these requirements is made as part of contract arrangements. Reference: www.tga.gov.au/docs/html/tgo/tgo69.htm Therapeutic Goods Order No.69 General Requirements for Labels for Medicines QS-SYS-P001 Version 2 Active: 5.10.11 Documents not printed on controlled paper are valid for the print date only Page 18 of 23 Author: R Makin SCGT - QUALITY MANUAL 15 Internal Audit The internal audit schedule is drawn up at the end of each calendar year for the forthcoming year by the Quality Manager with input from relevant staff. Audit management and reporting is performed using the Q-Pulse quality management system. It is the responsibility of the Quality Manager to maintain the overall use of the system including staff software training, implementing the audit schedule, timely completion of audits/ corrective actions and reporting the findings at the Quality Meeting and the Steering Committee Meeting. Audits are conducted periodically which cover both management and technical aspects of the quality system including all manufacturing processes. Audits also cover relevant national and international standards. Non-conformances to national and international requirements identified by internal or external auditors may require additional auditing of a policies, processes and/or procedures to investigate if the non-conformance is systematic. Audits are not to be performed by staff that have direct responsibility for the process they are auditing. This is/will be achieved by: Non-technical aspects of processes audited by the Quality Manager, Quality Officer and quality consultants; and Technical audits conducted by trained auditing staff that have the technical knowledge to understand a process but are not directly responsible for that process. References: QS-SYS-S009 (Internal Audit) QS-SYS-P009 (CAPA Policy) 16 Non-conformance A non-conformance is any deviation to testing, equipment, product, or quality system which may be detrimental to the manufacturing process. Non-conformances may be raised by staff, due to internal or external audits, complaints and client feedback. Where a non-conformance is raised the following is completed: The issue is recorded into Q-Pulse via the CA/PA module; The Quality Manager and relevant staff are notified via the email alerts of Q-Pulse; Action is taken to initially correct the issue and this action is recorded in Q-Pulse; An investigation is undertaken to assess the extent of the non-conformance; Where production will be effected, the Director and Production Manager are notified; Where necessary, production is ceased until the non-conformance has been investigated; Where potentially detrimental to the patient the patient and clinician are notified and product recalled prior to administration/transplant; Where the issue is identified post transplant the patient and the clinician are notified and a patient management plan established. In addition a IIMS report is completed; The root cause of the non-conformance is identified and recorded in Q-Pulse; Corrective action is taken and recorded to ensure the resolution of any systematic errors; and If necessary new procedures are established and implemented. Where a non-conformance is identified that casts doubt on the effectiveness of the quality system itself additional audits are conducted to identify the extent of the issue and to ensure the effectiveness of the resulting corrective action. QS-SYS-P001 Version 2 Active: 5.10.11 Documents not printed on controlled paper are valid for the print date only Page 19 of 23 Author: R Makin SCGT - QUALITY MANUAL References: QS-SYS-P009 (CAPA POLICY) QS-SYS-S008 (Product Recall) 17 Corrective Action/ Preventive Action Corrective Action The action taken to address a systemic problem is termed corrective action. Corrective action taken must be commensurate with the severity of a non-conformance raised. Corrective actions may be raised due to audits, external assessments, complaints and staff feedback. It is important that the root cause of the non-conformance is identified and eliminated via corrective action. This is more than just correction of the non-conformance but correction of any non-conformances of the systems contributing to the incident. The effectiveness of corrective actions should be monitored via audit to ensure the effectiveness of eliminating the root cause. Corrective actions are recorded in Q-Pulse in the CA/PA module. The responsibility for completion of corrective actions is defined in the Q-Pulse system. It is ultimately responsibility of the Quality Manager to monitor and ensure completion of corrective actions. Preventive Action Systems for proactively looking for potential non-conformances are termed preventive action. This is achieved by periodically auditing of policies and procedures, trend analysis of processes and reviews of quality control and facility monitoring data. All preventive actions are recorded in Q-Pulse and reviewed by the Quality Manager. Action items will be raised at the Quality Meeting. The responsibility for completion of preventive actions is defined in the Q-Pulse system. It is ultimately the responsibility of the Quality Manager to monitor and ensure completion of preventive actions. References: QS-SYS-P009 (CAPA Policy) QS-SYS-S008 (Product Recall) IIMS procedure -intranet 18 Quality Improvement The quality of the products manufactured by SCGT is ultimately indicated by their success in treating patients. Feedback from clinicians using the products is proactively sought. The number of successful and non-successful products, the microbial contamination rate and the number of series non-conformances is maintained for each unit and each staff member. 19 Management Review The suitability, adequacy and effectiveness of the quality system is assessed at the monthly Quality Meeting and the Steering Committee Meeting. Quality Meeting The Quality Meeting is held once a month. The production nominee or delegate from each unit must be present along with the Quality Manager and Quality Officer(s). QS-SYS-P001 Version 2 Active: 5.10.11 Documents not printed on controlled paper are valid for the print date only Page 20 of 23 Author: R Makin SCGT - QUALITY MANUAL The meeting is based on the Quality Meeting Agenda and the minutes and actions are recorded. The meeting is chaired by the Quality Manager and there is a nominated secretary. The intent of the meeting is to discuss quality system activities for the month, discuss audit findings, close out audits/ non-conformance reports/ corrective actions, delegate quality system tasks for the forthcoming month(s), discuss future needs of the quality system and to discuss issues to be raised with the Steering Committee. The last meeting of the year is focused on reviewing the whole quality management system (management review). SCGTs quality outcomes will be compared to the quality policy, mission and values. A management review report is provided at the Steering Committee Meeting. Steering Committee Meeting The Steering Committee Meeting is held monthly to discuss any issues that relate to the manufacture of products including expenditure, resourcing, use of the facilities and cGMP compliance. Membership of the Steering Committee is defined by the Terms of Reference. A secretary is nominated for the meetings. The minutes and actions of the meeting are recorded. The meeting addresses strategic, process and quality system issues. Major issues raised at the Quality Meeting are raised at this meeting for resolution. A report on the quality system is provided by the Quality Manager prior to each meeting. The action and prioritisation of the quality system issues are set at this meeting. Laboratory Meetings The individual unit all have a monthly minuted meeting. Issues raised at these meetings are tabled at the Quality Meeting and/or the Steering Committee Meeting. Email/ Internet The quality system documents, audits, corrective action and preventive action are accessed via a terminal internet server. Changes to documents, audit and action requests are made via Q-Pulse and emailed out to the staff on the distribution list. Important emails which have the potential to affect the product are recorded and saved on the hospital/ institute server. This information is back-up by the hospital/institute IT. References: QS-SYS-P002 (Management Review) QS-SYS-P003 (Westmead Research Hub Steering Committee TOR) 20 Ethics Accreditation/ Certification/ Licensing SCGT does not claim accreditation/ certification/ licensing for any process which has not been assessed to comply with the specified requirements/ regulations. The accreditation/ certification logo must only be used where the process is covered by the accreditation/ certification. This includes on websites, stationary, reports etc. Where logos are used the appropriate mandatory statements and logo orientation specified by the accreditation/ certification bodies must also be used. QS-SYS-P001 Version 2 Active: 5.10.11 Documents not printed on controlled paper are valid for the print date only Page 21 of 23 Author: R Makin SCGT - QUALITY MANUAL The Westmead Bone Marrow Transplant Laboratory is currently accredited to AS ISO 15189 by NATA. The scope of accreditation and accreditation status can be viewed at www.nata.asn.au. The rules of NATA accreditation (including logo use) are stated in NATA Rules which is also available at the website. Approvals Ethics approvals for the clinical trials performed by SCGT are reviewed by Human Research Ethics Committee’s in line with the TGA CTN and CTX approval system. The ethics committee reviewing approvals must comply with the Therapeutic Goods Act. References Human Research Ethics Committees and the Therapeutic Goods Legislation TGA Note for Guidance on Good Clinical Practice TGA 21 Complaints All complaints, whether received orally or in writing, should be recorded and investigated. There are five categories in which SCGT may receive a complaint. Each has established policies and procedures for resolution of the complaint. Patient /family The NSW Health Complaints Management Policy and Complaints Management Guidelines GL2006_023 are followed for all complaints made by patients and/or their families. All complaints are entered into the Incident Information Management System. This is accessible via the hospital intranet. Relating to a clinician The NSW Health Complaint or concern about Clinician Guidelines is followed. Service Contracts are set up with organisations using the SCGT facilities which specify the legal framework for disputes. Both CHW and WH have governance officers which may provide advice in such matters. Staff member Any complaint from a staff member should be resolved by the Director of the unit. Unresolved issues should be addressed by following NSW Health policy. Product Any complaint regarding a product manufactured by SCGT is notified on the first instance to the relevant Production Manager and the Quality Manager. In addition a corrective action report is initiated and the corrective action procedure followed. All significant complaints are raised with the Director of the unit and tabled at the Steering Committee meeting. Where the complaint raises concern about the relevant product or products the recall procedure will be followed. References: CHW and Westmead Hospital intranet QS-SYS-P009 (CAPA Policy) QS-SYS-S008 (Product Recall) QS-SYS-P001 Version 2 Active: 5.10.11 Documents not printed on controlled paper are valid for the print date only Page 22 of 23 Author: R Makin SCGT - QUALITY MANUAL 22 External users The facilities of SCGT may be leased to other biotechnology organisations. These organisations must comply with the facility and management requirements of SCGT as well as the policies of the hospital and/or institutes in which the facility is being leased. A clear contract of the responsibilities of each party is drawn up prior to use of the facility. Contracts should conform with the governance systems for each institution. A legal opinion may also be sought for some contracts. It is important that activities conducted by an external organisation do not invalidate the ability of SCGT to attain or maintain a TGA licence. If the organisation leasing the facility has its own quality system a certificate demonstrating licensing/ accreditation/ certification must be provided and left on file. An audit of the quality system may also be required. If the organisation does not have a recognised quality system SCGT may provide assistance in establishing a system. The external organisation may incur a cost for this service. Appendix 1 Changes to previous version Version 1 to Version 2 Included GCP references Update to document number Update of organisation chart Appendix 2 Relevant Standards and Guidelines AS/NZS ISO 9000 Quality system guide to selection and use AS/NZS ISO 9001 Quality systems – Model for quality assurance in design, development, production, installation and servicing AS1057 Cleanrooms and clean workstations AS3864 Medical refrigeration equipment – for the storage of blood and blood products ISO 14644 Cleanrooms and associated controlled environments Guide to GMP for medicinal products. Annex 1 – Manufacture of sterile medicinal products Note for Guidance on Good Clinical Practice TGA TGA Guidelines for sterility testing of Therapeutic Goods Council of Europe Publishing: Guide to the Preparation, use and quality assurance of blood components IATA Dangerous Goods Regulations, International Air Transport Association A WHO guide to good manufacturing practice (GMP) requirements Pharmaceutical Inspection Co-operation Scheme (PIC/S) Guide to Good Manufacturing Practice for Medicinal Products QS-SYS-P001 Version 2 Active: 5.10.11 Documents not printed on controlled paper are valid for the print date only Page 23 of 23 Author: R Makin