Reduced Scale Directions for Solution Preparation: Determination of
advertisement
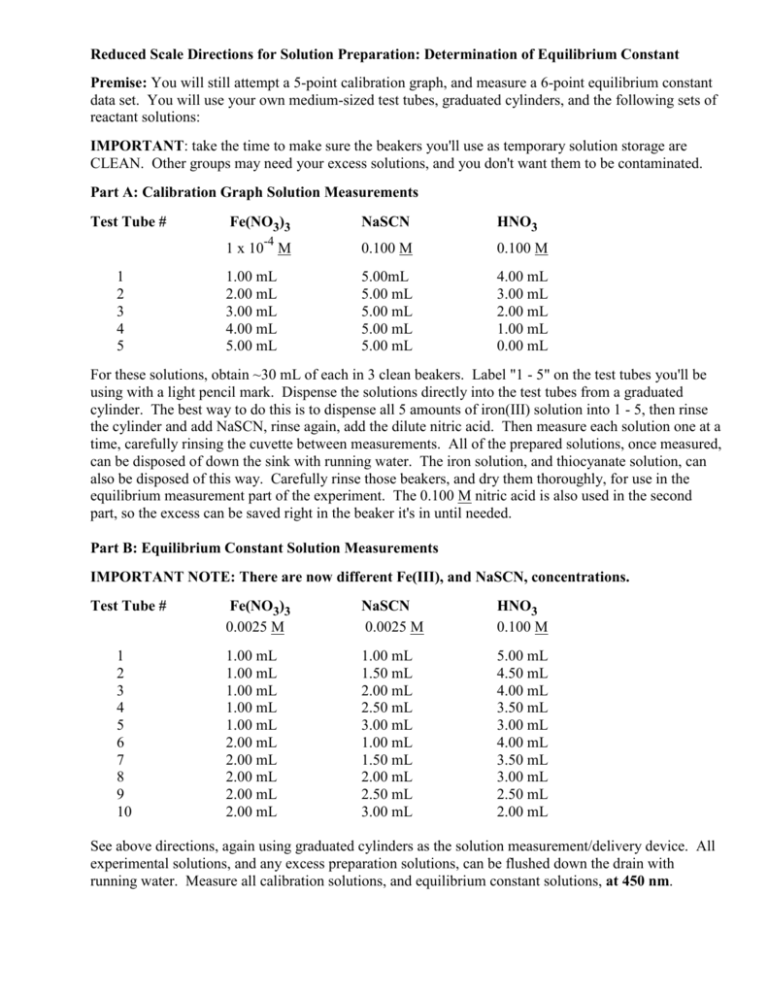
Reduced Scale Directions for Solution Preparation: Determination of Equilibrium Constant Premise: You will still attempt a 5-point calibration graph, and measure a 6-point equilibrium constant data set. You will use your own medium-sized test tubes, graduated cylinders, and the following sets of reactant solutions: IMPORTANT: take the time to make sure the beakers you'll use as temporary solution storage are CLEAN. Other groups may need your excess solutions, and you don't want them to be contaminated. Part A: Calibration Graph Solution Measurements Test Tube # 1 2 3 4 5 Fe(NO3)3 NaSCN HNO3 -4 1 x 10 M 0.100 M 0.100 M 1.00 mL 2.00 mL 3.00 mL 4.00 mL 5.00 mL 5.00mL 5.00 mL 5.00 mL 5.00 mL 5.00 mL 4.00 mL 3.00 mL 2.00 mL 1.00 mL 0.00 mL For these solutions, obtain ~30 mL of each in 3 clean beakers. Label "1 - 5" on the test tubes you'll be using with a light pencil mark. Dispense the solutions directly into the test tubes from a graduated cylinder. The best way to do this is to dispense all 5 amounts of iron(III) solution into 1 - 5, then rinse the cylinder and add NaSCN, rinse again, add the dilute nitric acid. Then measure each solution one at a time, carefully rinsing the cuvette between measurements. All of the prepared solutions, once measured, can be disposed of down the sink with running water. The iron solution, and thiocyanate solution, can also be disposed of this way. Carefully rinse those beakers, and dry them thoroughly, for use in the equilibrium measurement part of the experiment. The 0.100 M nitric acid is also used in the second part, so the excess can be saved right in the beaker it's in until needed. Part B: Equilibrium Constant Solution Measurements IMPORTANT NOTE: There are now different Fe(III), and NaSCN, concentrations. Test Tube # 1 2 3 4 5 6 7 8 9 10 Fe(NO3)3 0.0025 M NaSCN 0.0025 M HNO3 0.100 M 1.00 mL 1.00 mL 1.00 mL 1.00 mL 1.00 mL 2.00 mL 2.00 mL 2.00 mL 2.00 mL 2.00 mL 1.00 mL 1.50 mL 2.00 mL 2.50 mL 3.00 mL 1.00 mL 1.50 mL 2.00 mL 2.50 mL 3.00 mL 5.00 mL 4.50 mL 4.00 mL 3.50 mL 3.00 mL 4.00 mL 3.50 mL 3.00 mL 2.50 mL 2.00 mL See above directions, again using graduated cylinders as the solution measurement/delivery device. All experimental solutions, and any excess preparation solutions, can be flushed down the drain with running water. Measure all calibration solutions, and equilibrium constant solutions, at 450 nm.











