Iron (III)
advertisement

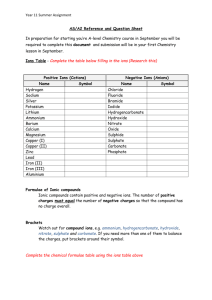
Ions to Formulae and Equations Ions to formulae In the correct formula of a compound the charges on the ion must cancel out. Using the ion table attached and the set of rule cards below the excercises, you can turn the name of a compound into its formula. Start with the name :- sodium write in the ions :balance the charges :write the formula :- chloride iron III oxide lithium sulphate Cheats Method :- sodium write in the ions :swap the numbers :cancel down :- chloride iron III oxide lithium sulphate Use either method to find the formulae of the following compounds. Exercise 1 a. b. c. d. e. f. Sodium fluoride Lithium iodide Magnesium sulphide Calcium oxide Aluminium nitride Iron (III) phosphide g. h. i. j. k. l. Magnesium chloride Sodium sulphide Aluminium iodide Lithium nitride Magnesium nitride Aluminium oxide g. h. i. j. k. l. Iron(II) sulphate Hydrogen chloride Silver nitrate Sodium hydrogencarbonate Lead (II) carbonate Ammonium hydroxide Exercise 2 a. b. c. d. e. f. Potassium nitrate Sodium hydroxide Magnesium sulphate Calcium carbonate Aluminium phosphate Ammonium chloride In the next exercise you may need to use brackets in the formulae. We use brackets around polyatomic ions (sulphate, nitrate, ammonium) whenever we need two or more of them ; e.g. sodium hydroxide, NaOH copper hydroxide, Cu(OH)2 aluminium hydroxide, Al(OH)3 Exercise 3 a. b. c. d. e. f. Copper (II) nitrate Sodium sulphate Calcium hydroxide Sodium hydrogensulphate Aluminium nitrate Potassium hydrogen phosphate g. h. i. j. k. l. Iron (III) sulphate Copper (II) ethanoate Ammonium carbonate Calcium phosphate Iron (III) carbonate Ammonium sulphate Ions to Formulae and Equations Now balance some simple equations. Write the word equation and the write in the formulae underneath it Sodium hydroxide + hydrochloric acid sodium chloride + water NaOH + HCl NaCl + H2O In a balanced equation there will be the same number of atoms of each element on both sides of the arrow. In this example there is one sodium atom in the sodium hydroxide and one in the sodium chloride. There is one atom of hydrogen on each side also. Count the hydrogens and the chlorine. Exercise 4 1. Calcium carbonate calcium oxide + carbon dioxide 2. Hydrochloric acid + sodium hydroxide sodium chloride + water 3. Magnesium + sulphuric acid magnesium sulphate + hydrogen gas 4. Copper sulphate + zinc zinc sulphate + copper 5. Iron II oxide + calcium calcium oxide + iron In more difficult equations you need to multiply some of the formulae to balance the elements; Copper + oxygen gas copper oxide Cu + O2 CuO Start with what changes; there are 2 O’s in O2 and only one in CuO. To balance it you need 2 O’s in the products. Don’t be tempted to write CuO2, you have found the formula and you can’t change it. Instead double the CuO Cu + O2 2CuO Now you have balanced the oxygen, you have 2 O’s in O2 and in the 2CuO But you now have 2Cu’s in the products but only one in the reactants, so double the Cu 2Cu + O2 2CuO Quickly double check each element, all done. Ions to Formulae and Equations Try these harder ones; hydrogen gas is H2, oxygen gas is O2 Exercise 5 1. Magnesium + oxygen gas magnesium oxide 2. Aluminium + oxygen gas aluminium oxide 3. Zinc + hydrochloric acid zinc chloride + hydrogen gas 4. Copper oxide + nitric acid copper nitrate + water 5. Calcium carbonate + hydrochloric acid calcium chloride + water + carbon dioxide gas 6. Sodium + oxygen gas sodium oxide 7. Sodium hydroxide + sulphuric acid sodium sulphate + water 8. Sodium + water sodium hydroxide + hydrogen gas 9. Aluminium + Iron (III) oxide aluminium oxide + iron 10. Magnesium nitrate magnesium oxide + oxygen gas + nitrogen dioxide (NO2) The last one is difficult, if you can’t balance it first time through, try starting with two magnesium nitrates. Ions to Formulae and Equations Answers Exercise 1 a. b. c. d. e. f. Sodium fluoride Lithium iodide Magnesium sulphide Calcium oxide Aluminium nitride Iron (III) phosphide NaF LiI MgS CaO AlN FeP g. h. i. j. k. l. Magnesium chloride Sodium sulphide Aluminium iodide Lithium nitride Magnesium nitride Aluminium oxide KNO3 NaOH MgSO4 CaCO3 AlPO4 NH4Cl g. h. i. j. k. l. Iron(II) sulphate Hydrogen chloride Silver nitrate Sodium hydrogencarbonate Lead (II) carbonate Ammonium hydroxide MgCl2 Na2S AlI3 Li3N Mg3N2 Al2O3 Exercise 2 a. b. c. d. e. f. Potassium nitrate Sodium hydroxide Magnesium sulphate Calcium carbonate Aluminium phosphate Ammonium chloride FeSO4 HCl AgNO3 NaHCO3 PbCO3 NH4OH In the next exercise you may need to use brackets in the formulae. We use brackets around polyatomic ions (sulphate, nitrate, ammonium) whenever we need two or more of them ; e.g. sodium hydroxide, NaOH copper hydroxide, Cu(OH)2 aluminium hydroxide, Al(OH)3 Exercise 3 a. b. c. d. e. f. Copper (II) nitrate Sodium sulphate Calcium hydroxide Sodium hydrogensulphate Aluminium nitrate Potassium hydrogenphosphate Cu(NO3)2 Na2SO4 Ca(OH)2 NaHSO4 Al(NO3)3 K2HPO4 g. h. i. j. k. l. Iron (III) sulphate Copper (II)ethanoate Ammonium carbonate Calcium phosphate Iron (III) carbonate Ammonium sulphate Fe2(SO4)3 Cu(CH3COO)2 (NH4)2CO3 Ca3(PO4)2 Fe2(CO3)3 (NH4)2SO4 Ions to Formulae and Equations Exercise 4 1. Calcium carbonate calcium oxide + carbon dioxide CaCO3 CaO + CO2 2. Hydrochloric acid + sodium hydroxide sodium chloride + water HCl + NaOH NaCl + H2O 3. Magnesium + sulphuric acid magnesium sulphate + hydrogen gas Mg + H2SO4 MgSO4 + H2 4. Copper sulphate + zinc zinc sulphate + copper CuSO4 + Zn ZnSO4 + Cu 5. Iron II oxide + calcium calcium oxide + iron FeO + Ca CaO + Fe Exercise 5 1. Magnesium + oxygen gas magnesium oxide Mg + O2 MgO 2. Aluminium + oxygen gas aluminium oxide 4Al + 3O2 2Al2O3 3. Zinc + hydrochloric acid zinc chloride + hydrogen gas Zn + 2HCl ZnCl2 + H2 4. Copper oxide + nitric acid copper nitrate + water CuO + 2HNO3 Cu(NO3)2 + H2O 5. Calcium carbonate + hydrochloric acid calcium chloride + water + carbon dioxide gas CaCO3 + 2HCl CaCl2 + H2O + CO2 6. Sodium + oxygen gas sodium oxide 4Na + O2 2Na2O 7. Sodium hydroxide + sulphuric acid sodium sulphate + water 2NaOH + H2SO4 Na2SO4 + 2H2O 8. Sodium + water sodium hydroxide + hydrogen gas 2Na + 2H2O 2NaOH + H2 9. Aluminium + Iron (III) oxide aluminium oxide + iron 2Al + Fe2O3 Al2O3 + 2Fe 10. Magnesium nitrate magnesium oxide + oxygen gas + nitrogen dioxide (NO2) 2Mg(NO3)2 2MgO + O2 + 4NO2 OR Mg(NO3)2 MgO + 1/2O2 + 2NO2 Ions to Formulae and Equations Rule 1 Write the symbols for the cation and the anion e.g. Na & Cl Ions to Formulae and Equations RULE 2 Determine the charge on the cation and anion from their position on the periodic table + Na & - Cl Ions to Formulae and Equations Deducing the charge on the ion from the periodic table; how does it work? Group I has only one electron in its outer shell, so when it loses that electron it will then have a +1 charge. Groups II and III lose two and three electrons in their outer shells respectively to become charged +2 and +3. Group IV can go either way. It can either lose or gain four electrons. It rarely forms ions, though. Group V with its five electrons in its outer shell is when things change. Group V will gain three electrons to have a -3 charge. Group VI gains two electrons to have a -2 charge. Group VII has seven electrons in its outer shell, so it gains one electron to have a -1 charge Ions to Formulae and Equations RULE 3 If the cation has a Roman numeral after it, that is the charge on that cation. Cations receive Roman numerals when they can take more than one ionic form, e.g. Iron (III) Oxide Ions to Formulae and Equations RULE 4 Write the two symbols together, and determine how to make the compound neutral by finding the lowest common multiple of the charges on each ion. Then figure out how atoms of each element are needed to make that charge e.g. NaCl Ions to Formulae and Equations Let's try working out Sodium Oxide 1. Write Na and O. 2. Put in the charges so write Na+ and O2-. 3. Na+O2-, the lowest common multiple is 2. To get a charge of 2 on sodium (which has a +1 charge), you need to multiply it by 2 (1 × 2 = 2). So you will have two atoms of sodium. To get a charge of 2 on the oxygen, you multiply it by 1 (2 × 1 = 2). So, just one O. Therefore we write Na2O Try working out Iron (III) Oxide Ions to Formulae and Equations Polyatomic ions Writing formulas for polyatomic ionic compounds isn't hard when you know the formula for the polyatomic ion. There are many tables available that show the formula for common polyatomic ions and the charge on them. Over time, you will easily memorise the ones you use most. Use the same steps as when you name simple ionic compounds, but treat the polyatomic ion as one unit. Ions to Formulae and Equations Let's try an example. Iron (III) Chromate. 1. Write Fe and CrO4. 2. Put in the charges, so write Fe3+ and CrO42- 3. Balance the charges and you end up with Fe2(CrO4)3 . The lowest common multiple of the +3 charge on the iron and the -2 charge on the chromate is 6. So, you need two iron ions (3 × 2 = 6) and three chromate ions (2 × 3 = 6) to make a neutral compound. Fe2(CrO4)3. The three outside the brackets indicates that everything inside those brackets is multiplied by that number. Try working out Ammonium Phosphate. Ions to Formulae and Equations Writing & balancing equations A chemical equation describes what happens in a chemical reaction. The equation identifies the reactants (starting materials) and products (resulting substance), their formulae, phases (solid, liquid, gas), and the amount of each substance. o Write the unbalanced equation. o Chemical formulas of reactants are listed on the left-hand side of the equation. o Products are listed on the right-hand side of the equation. o Reactants and products are separated by putting an arrow between them to show the direction of the reaction. o Get the same number of atoms of every element on each side of the equation. o Once one element is balanced, proceed to balance another, and another, until all elements are balanced. o Balance chemical formulae by placing coefficients in front of them. Do not add subscripts, because this will change the formulae. Ions to Formulae and Equations o Example Look at the equation below and see which elements are not balanced. In this case, there are two oxygen atoms on the lefthand side of the equation and only one on the right-hand side. Correct this by putting a coefficient of 2 in front of water: SnO2 + H2 → Sn + H2O This puts the hydrogen atoms out of balance. Now there are two hydrogen atoms on the left and four hydrogen atoms on the right. To get four hydrogen atoms on the left, add a coefficient of 2 for the hydrogen gas. Remember, coefficients are multipliers, so if we write 2 H2O it denotes 2x2=4 hydrogen atoms and 2x1=2 oxygen atoms. SnO2 + 2 H2 → Sn + 2 H2O The equation is now balanced. Be sure to double-check your math! Each side of the equation has 1 atom of Sn, 2 atoms of O, and 4 atoms of H.