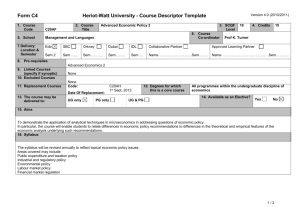
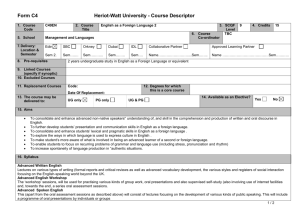
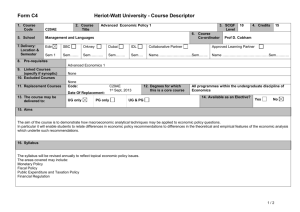
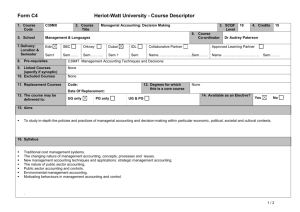
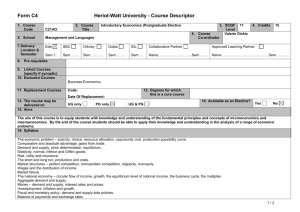
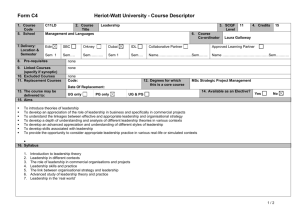
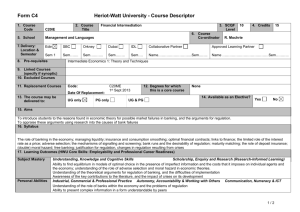
Table 1S. Primer information. Table 2S. MPN patient information. Fig
advertisement

1 2 Table 1S. Primer information. 3 4 Table 2S. MPN patient information. 5 6 Fig 1S. Volcano plot of miRNA deregulation in MPN patient CD34+ cells. Yellow: 7 miRNAs not expressed in controls but expressed in MPN patients. Red: miRNAs 8 expressed in controls but not expressed in MPN patients. Blue: miRNAs with 9 deregulated expression level. 10 11 Fig 2S. miRNA array validation in MPN patient CD34+ by qRT-PCR. Same RNA 12 samples used the Taqman miRNA array was utilized in single qRT-PCR to confirm the 13 expression levels of miR-22*, miR-133b and miR-9. 14 15 Fig 3S. Heat map represents the Pearson correlation between patient and control 16 samples based on raw Ct values. High correlation coefficient indicates similar miRNA 17 expression profile. 18 19 Table 3S. List of deregulated miRNAs in MPN CD34+ cells with known function in 20 hematopoietic systems. 21 22 Table 4S. Commonly deregulated miRNAs in MPN CD34+ cells and MPN 23 granulocytes (p<0.01). A subset of the deregulated miRNAs are also differentially 24 expressed in an independent miRNA array study using MPN patient granulocytes 25 (n=39, 25 PV, 14 ET). 26 27 Table 5S. 27 miRNAs were significantly deregulated in PV (n=3) compared to 28 controls (n=4) (p<0.01). 29 30 Table 6S. 116 miRNAs were significantly deregulated in ET (n=3) compared to 31 controls (n=4) (p<0.01). 32 33 Fig 4S. Identification of JAK2V617F-independent miRNAs. (A) HEL cells were 34 treated with JAK inhibitor (2uM) (Calbiochem, Rockland, MA) or DMSO. Number of live 35 cells was determined by Trypan Blue at 12 hrs (left panel). miRNA was extracted at 12 36 hrs and quantified by qRT-PCR (right panel). Data are expressed as the mean ± SEM 37 of biological triplicates. (B) HEL cells were treated with JAK inhibitor (2uM) 38 (Calbiochem, Rockland, MA) or DMSO. Number of live cells was determined by Trypan 39 Blue at 24 hrs (left panel). miRNA was extracted at 24 hrs and quantified by qRT-PCR 40 (right panel). Data are expressed as the mean ± SEM of biological triplicates. 41 42 Fig 5S. miRNA responsiveness to manipulation of JAK2 activity in TF1 cells and 43 JAK2V617F knock-in mice. (A) The JAK2 lentiviral construct uses a two-promoter 44 system, with GFP driven by the EF1 promoter and A2 driving JAK2 (gift of Dr. Carl 45 June, University of Pennsylvania). Human wild-type JAK2 and JAK2V617F cDNA was 46 cloned downstream of the A2 promoter using NheI and SalI sites. (B) JAK2WT and 47 JAK2V617F viral particles were produced by transient transfection of 293T cells with 48 EF1-GFP-JAK2WT/V617F, pRSV-REV, pMDLg/RRE and pGMV/VSVG constructs 49 using Fugene6 (Roche). After 24h, medium was replaced with fresh DMEM, and virus 50 was collected at 48h. TF-1 cells were stably transduced with control 2A lentivirus or 51 JAK2V617F. Infected TF-1 cells were cultured in the absence of cytokines for 22 hrs 52 before mRNA was extracted and quantified for JAK2 expression by RT-PCR. (C) Cells 53 were cultured with or without cytokines for 4 days and cell density was determined by 54 MTT assay (right panel). (D) Total RNA was extracted from the stably transduced TF-1 55 cells and miRNA expression levels were determined by RT-PCR. Data are expressed 56 as the mean ± SEM of technical triplicates(*p<0.05). (E) After euthanization, the femurs 57 of JAK2 transgenic mice were flushed with PBS RBCs were removed with lysis buffer 58 (Sigma-Aldrich, St. Louis, MO), and remaining cells were washed and pelleted. Total 59 RNA was extracted using the MirVana kit, and miRNA levels were evaluated by qRT- 60 PCR. 61 62 Fig 6S. miR-433 expression is not changed during myelopoiesis and 63 megakaryopoiesis from BM CD34+ cells. (A) CD34+ cells were cultured in SFEM 64 supplemented with cytokines to promote myeloid (50ng/ml SCF, 10ng/ml IL-3, 10ng/ml 65 IL-6 and 10ng/ml GMCSF) lineages for 12 days. miRNA was extracted on days 3, 6, 9 66 and 12 and quantified by RT-PCR. Data are expressed as the mean ± SEM of 67 biological triplicates. (B) CD34+ cells were cultured in SFEM supplemented with 68 cytokines to promote and megakaryocytic (50ng/ml TPO, 10ng/ml IL-6, 10ng/ml IL-3) 69 lineages for 12 days. miRNA was extracted on days 3, 6, 9 and 12 and quantified by 70 RT-PCR. Data are expressed as the mean ± SEM of biological triplicates. 71 72 Fig 7S. miR-433 expression validation. (A) Validation of miR-433 upregulation in 73 MPN patient BM CD34+ cells by RT-PCR. Un-amplified RNA from patient and control 74 BM CD34+ cells was used for RT-PCR quantification. (B) CD34+ cells were stably 75 transduced with pmiR empty vector, pmiR scrambled control, or pmiR-433 lentivirus, 76 and cultured in erythroid liquid culture for 9 days. miRNA was extracted on day 8 and 77 miR-433 expression level was assessed by RT-PCR. Due to the low cell number, cells 78 from biological triplicates were pooled together for RT-PCR, and data are expressed as 79 the mean ± SEM of technical triplicates. (C) CD34+ cells were stably transduced with 80 pmiR empty vector, pmiR scrambled control, or pmiR-433 lentivirus, and cultured in 81 mythylcellulose supporting erythroid and myeloid colony formation for 14 days. miRNA 82 was extracted on day 14 and miR-433 expression level was assessed by RT-PCR. 83 84 Figure 8S. miR-433 negatively regulates myeloid and CFU-MK colony formation. 85 (A) CD34+ cells were stably transduced with miR-433 or scrambled control lentivirus. 86 4x103 GFP sorted cells were seeded in 3ml of Methocult® H4434 medium (Stem Cell 87 Technologies) and duplicate 1.4ml aliquots were plated into 35-mm dishes (Corning, 88 Corning, NY). Three independent experiments were performed, and colony number 89 was assessed on D14. miR-433 colony formation is presented as a relative ratio to 90 pmiR-scr, and data are expressed as the mean ± SEM of biological triplicates (p<0.05). 91 (B) CD34+ cells were stably transduced with miR-433 or scrambled control lentivirus, 92 and plated in Megacult-C supporting CFU-MK colony formation. Two independent 93 experiments were performed. Slides were fixed on D12 and stained within three days. 94 miR-433 colony formation is presented as a relative ratio to pmiR-scr, and data are 95 expressed as the average ± SEM of biological duplicates. (C) CD34+ cells were stably 96 transduced with miRZIP-433 or miRZIP scrambled control lentivirus, and plated in 97 methylcellulose supporting myeloid colony formation. Three independent experiments 98 were performed, and colony number was assessed on day 14. miRZIP-433 colony 99 formation is presented as a relative ratio to miRZIP-scr, and data are expressed as the 100 mean ± SEM of biological triplicates (p<0.05). (D) CD34+ cells were stably transduced 101 with miRZIP-433 or scrambled control lentivirus, and plated in Megacult-C supporting 102 CFU-MK colony formation. Two independent experiments were performed. Slides were 103 fixed on day 12 and stained within three days. miRZIP-433 colony formation is 104 presented as a relative ratio to miRZIP-scr, and data are expressed as the average ± 105 SEM of biological duplicates. 106 107 Fig 9S. An independent experiment to confirm miR-433 silencing in ABM CD34+ 108 promotes erythroid proliferation. CD34+ cells were stably transduced with miRZIP- 109 433 miRNA inhibitor or miRZIP scrambled control lentivirus, and cultured in erythroid 110 liquid culture for 9 days. The number of live cells in each sample was counted using 111 Countess Automated Cell Counter on days 3, 6 and 9, and are expressed in a 112 cumulative growth curve. 113 114 Table 7S. Genes deregulated in miR-433 overexpressing CD34+ cells and 115 predicted to be direct targets of miR-433 by miRecords (functional information 116 from GeneCards®). 117 118 Fig 10S. GBP2 Illumina array raw intensity. 119 120 Fig 11S. GBP2 overexpression and knockdown confirmation. (A) TF-1 were stably 121 transduced with GBP2 or empty vector control lentivirus, and cultured in 1U/ml EPO. 122 Total RNA was extracted on day 5 and the expression level of GBP2 was measured by 123 qRT-PCR. Average expression is expressed as the mean ± SEM of biological 124 triplicates. (B) TF-1 were stably transduced with a shRNA targeting GBP2 or scrambled 125 shRNA control lentivirus, and cultured in 1U/ml EPO. Total RNA was extracted on day 126 5 and the expression level of GBP2 was measured by qRT-PCR. Average expression 127 is expressed as the mean ± SEM of technical triplicates. 128 129 Fig 12S. MMP-9 expression in TF-1 cells with GBP2 overexpression or 130 knockdown. (A) Total RNA was extracted from TF-1 cells stably overexpressing GBP2 131 or empty vector. 132 expressed as the mean ± SEM of technical triplicates. (B) Total RNA was extracted 133 from TF-1 cells stably transduced with GBP2 shRNA or scrambled control. MMP-9 134 expression level was measured by qRT-PCR. Data is expressed as the mean ± SEM of 135 technical triplicates. 136 MMP-9 expression level was measured by qRT-PCR. Data is 137 Fig 13S. FACS analysis of TF-1 cells with GBP2 overexpression or knockdown. 138 (A) FACS analysis was performed using stably transduced TF-1 cells overexpressing 139 GBP2. Cells were cultured in 2ug/ml GMCSF as baseline for EPO differentiation 140 analysis. Surface markers CD71 (APC) and CD235a (PE) were measured. Data are 141 expressed in biological replicates. (B) FACS analysis was performed using stably 142 transduced TF-1 cells overexpressing GBP2, and cultured in 1U/ml EPO. 143 Differentiation was determined by measuring surface markers CD71 (APC) and 144 CD235a (PE) on day 6. Data are expressed in biological triplicates. (C) FACS analysis 145 was performed using TF-1 cells with reduced GBP2 expression, and cultured in 1U/ml 146 EPO. Differentiation was determined by measuring surface markers CD71 (APC) and 147 CD235a (PE) on day 6. Data are expressed in biological triplicates. 148 149 Fig 14S. A separate shRNA targeting GBP2 confirms that reduced GBP2 level 150 inhibits proliferation and accelerates erythroid differentiation in TF-1. (A) TF-1 151 were stably transduced with shRNA’ GBP2 or scrambled shRNA control lentivirus, and 152 cultured in 1U/ml EPO. Total RNA was extracted on day 5 and the expression level of 153 GBP2 was measured by qRT-PCR. Average expression is expressed as the mean ± 154 SEM of biological triplicates. (B) TF-1 cells stably transduced shRNA’ GBP2 or 155 scrambled control lentivirus were cultured in 1U/ml EPO for 6 days. The number of live 156 cells in each sample was counted using Countess Automated Cell Counter on days 3 157 and 6, and are expressed in a cumulative growth curve. Data are expressed as the 158 mean ± SEM of biological triplicates (*P<0.05). (C) FACS analysis was performed 159 using TF-1 cells with reduced GBP2 expression, and cultured in 1U/ml EPO. 160 Differentiation was determined by measuring surface markers CD71 (APC) and 161 CD235a (PE) on day 6. Data are expressed in biological triplicates.